Introduction
Helicobacter pylori (H. pylori) is a gram-negative bacterium that infects 50% of the world’s population. It is invariably associated with gastritis and may lead to severe outcomes, including peptic ulcer disease, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma [1-3]. In accordance with the 2015 Kyoto consensus, Maastricht VI/Florence report states that H. pylori is considered an infectious disease and is now included as a specific disease entity in the new International Classification of Diseases 11th Revision (ICD11) [1, 4].
The prevalence of H. pylori varies across different geographical regions, with Western Europe being distinguished by one of the lowest reported occurrences [2, 3, 5]. Nevertheless, Portugal is highlighted as one of the countries facing a substantial burden, with a prevalence exceeding 60%, reaching 84% in the northern area [6, 7].
Optimal management of H. pylori infection is challenging due to the escalating antimicrobial bacterial resistance [8]. A Portuguese meta-analysis reveals that primary resistance rate to metronidazole (M) ranges between 10 and 34%, to clarithromycin (C) is 32%, and dual resistance to both antibiotics reaches 5% [6]. According to current guidelines, in areas with a high (>15%) C resistance rate, the recommended first-line empirical treatment consists of bismuth-containing quadruple therapy with bismuth salts (B), M, tetracycline (Tc), and a proton-pump inhibitor (PPI) (quadruple-BMTc), or non-B concomitant therapy with C, amoxicillin (A), M, and a PPI (concomitant-CAM). Standard triple therapy (triple-CA) is the recommended regimen in areas with low C resistance rate [1]; thus, it is no longer considered an acceptable treatment in the adult Portuguese population [6, 9].
H. pylori gastritis has the potential to progress to gastric cancer [10]. As Portugal exhibits some of the highest gastric cancer incidence, optimizing the H. pylori eradication rate becomes mandatory [11].
A recommended anti-H. pylori regimen is presently described as one that consistently achieves an eradication rate of at least 90% [12]. The overall effectiveness can be classified as excellent (>95% success), good (>90%success), borderline acceptable (85-89% success), or unacceptable (<85% success) [12]. The European Registry on Helicobacter pylori Management (Hp-EuReg) is a platform that aggregates information on real-world H. pylori management across a multitude of European countries, in order to homogenize and provide a large-scale perspective on the current state of the clinical practice [13]. Our objective was to describe the management of H. pylori infection by evaluating the effectiveness and safety of first-line empirical treatments, in Portugal by gastroenterologists within a real clinical practice setting.
Materials and Methods
This manuscript represents the cohort of Portuguese patients within the Hp-EuReg, an international, multi-centre, non-interventional registry, recording information of H. pylori infection management since May 2013, promoted by the European Helicobacter and Microbiota Study Group (EHMSG, https://www.helicobacter.org). The registry is currently receiving input from 38 European countries with the collaboration of over 300 centres. The study is still recruiting patients, without any limitation or stopping point defined; so, there is no applicable sample size. The detailed information regarding Hp-EuReg can be found in the published protocol [14].
The study was conducted according to ethical guide-lines of the 1975 Declaration of Helsinki, and Hp-EuReg protocol was approved by the Ethics Local Committee of La Princesa University Hospital of Madrid (Spain), registered at ClinicalTrials.gov with code NCT02328131. The current manuscript follows the recommendation for Reporting of Observational Studies in Epidemiology (STROBE) statement [15].
Participants
Portuguese adults diagnosed with H. pylori infection and included in the Hp-EuReg between May 2013 and December 2022 were considered. Participants were in-cluded by gastroenterologists if submitted to any attempt of H. pylori treatment, without any exclusion criteria.
Information containing the patient’s demographic and clinical data, diagnostic methods before and after treatment, previous eradication attempts, treatment regimen details, and its associated eradication rate and compliance was collected using an electronic case report form. Additionally, safety was assessed through the reporting of adverse events (AEs) and any required treatment interruptions due to these events. The information was registered at the Research Electronic Data Capture (RED-Cap) application hosted at “Asociación Española de Gastroenterología,” Madrid, Spain (AEG; https://www.aegastro.es; accessed on January 1, 2024), a non-profit scientific and medical society of gastroenterologists whose goal is to promote research.
Data Management
After the data extraction, the database was reviewed for inconsistencies and subsequent data cleaning. The data quality review process evaluated whether the study selection criteria had been met and whether data were correctly collected, ensuring the study was conducted according to the highest scientific and ethical standards. Data discordances were resolved by querying the investigators and through group emailing. Prior to the statistical analyses, the Hp-EuReg Scientific Director ensured coherence, data quality, and scientific integrity.
Statistical Analyses
Variable Categorization and Definition
To ease the synthesis of information, our analysis focused on the 4 most frequently used treatments, defined as follows: triple-CA, sequential-CAM, concomitant-CAM, and quadruple-BMTc. Compliance with treat-ment was defined as taking at least 90% of the prescribed drugs.
The analysis was stratified by length of treatment (at least 7, 10, or 14 days) and PPI dose (low, standard, and high). For PPI doses, low dose was defined as 4.5-27 mg omeprazole equivalents, twice daily; and high dose was defined as 54-128 mg omeprazole equivalents, twice daily; all other doses were considered as standard [16].
Data Analysis
In order to evaluate the effectiveness of the different regimens, we performed an analysis in 3 groups of pa-tients: an intention-to-treat (ITT) analysis, regarding data with a 6-month period follow-up and considering as treatment failures the patients lost to follow-up (i.e., the result of the confirmatory test after the eradication treatment was not available); a modified ITT (mITT) analysis, including patients that had completed the follow-up (i.e., the result of the confirmatory test after the eradication treatment was available - success or failure); regardless of whether they had complied with treatment or not, in order to closely align with everyday real clinical practice; and a per-protocol (PP) analysis included pa-tients who had completed follow-up and were compliant (i.e., had taken ≥90% of the prescribed drugs). In statistical analysis, continuous data were presented as the mean and standard deviation, while qualitative data were presented as the absolute relative frequencies, displayed as percentages (%) and corresponding 95% confidence intervals. Statistical analysis was performed using IBM SPSS Statistics (version 25). The selected level of statistical significance was p < 0.05.
Results
Overall, 700 cases were included in the Hp-EuReg in Portugal between 2013 and 2022. Patients were enrolled via outpatient clinic at five distinct medical facilities throughout Portugal, only by gastroenterologists: two centres provided 98% of cases - Instituto Português de Oncologia de Coimbra and Centro Hospitalar e Universitário do Porto, while the other cases were provided by Instituto Português de Oncologia do Porto, Centro Hospitalar de Vila Nova de Gaia e Centro Hospitalar de Tondela-Viseu.
Baseline Characteristics
There were 59% of female patients (n = 410), with a mean age of 54 ± 15 years (range 15-84), and 99% were Caucasian (n = 696). Allergy to antibiotics was registered in 4.4% (n = 31), most of them to penicillin 4% (n = 28). The main indications for H. pylori eradication were dyspeptic symptoms (47%), duodenal ulcer (3.7%), gastric ulcer (3.7%), and other causes (39%) that included anaemia and atrophic gastritis.
H. pylori diagnosis, before eradication treatment, was mainly made through invasive strategies: histology 90%(n = 627), culture 2.3% (n = 16), and rapid urease test 0.3% (n = 2); the most used non-invasive test was 13C urea breath test 4.9% (n = 34). The eradication confirmatory test was performed in 93% of the cases (n = 649), with 7.3% of the patients lost to follow-up (n = 51). The diagnostic methods after eradication were also analysed, with 13C urea breath test in 64% (n = 448), 14C urea breath test in 23% (n = 159), and histology in 5.9% (n = 41) being the most frequently used confirmatory test choices.
Prescriptions
Regarding the therapeutic regimens, the most frequently prescribed schemes were the quadruple therapy, including the concomitant-CAM in 32% (n = 226) and the quadruple-BMTc in 31% (n = 215), followed by triple-CA in 27% (n = 186). Other unfrequently used prescriptions such as concomitant-CAM for 10 days or quadruple-BMTc for 14 days had no more than 17 cases and were not eligible for statistical analysis.
The prescription strategy of the PPIs was also analysed, with esomeprazole being the leading choice 37% (n = 257), followed by pantoprazole 29% (n = 199), rabeprazole 20%(n = 141), omeprazole 13% (n = 88), and lansoprazole 2%(n = 12). In 98% of the cases, the PPIs were prescribed twice a day and once a day in 2%. The low PPI dosage (i.e., 20 mg omeprazole equivalent twice daily) was the most used PPI prescription in 40% (n = 276) cases.
First-Line Therapy Effectiveness
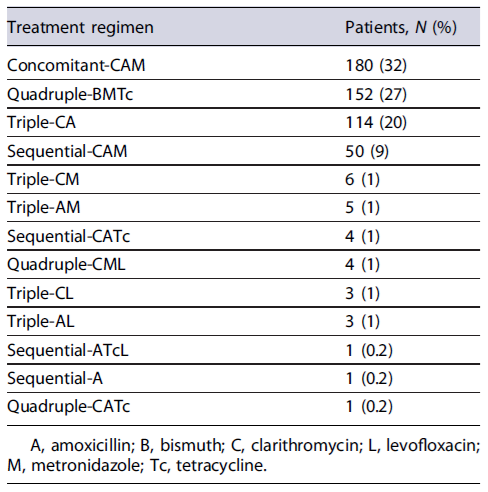
From the 700 studied cases, 81% (n = 564) received a first-line empirical treatment, and out of 16 different therapeutic regimens, 13 were prescribed as first-line therapy, reported in Table 1. Patients who underwent first-line eradication therapy were mainly prescribed with concomitant-CAM (n = 180, 32%), quadruple-BMTc (n = 152, 27%), triple-CA (n = 114, 20%), and sequential-CAM (n = 50, 9%).
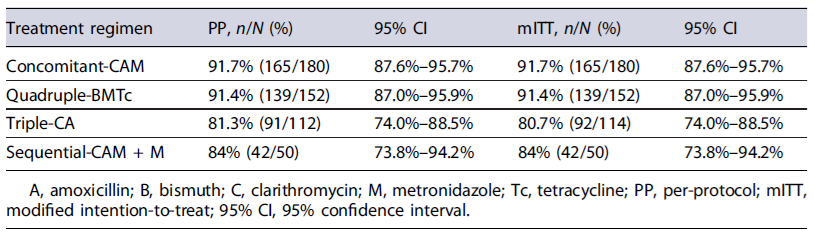
The mITT and PP effectiveness of each first-line treatment regimen are presented in Table 2. After confirming eradication with a validated diagnostic test, the overall effectiveness of H. pylori first-line eradication therapy was 87% by both mITT and PP. Concomitant-CAM (92%) and quadruple-BMTc (91%) obtained optimal (>90%) effectiveness.
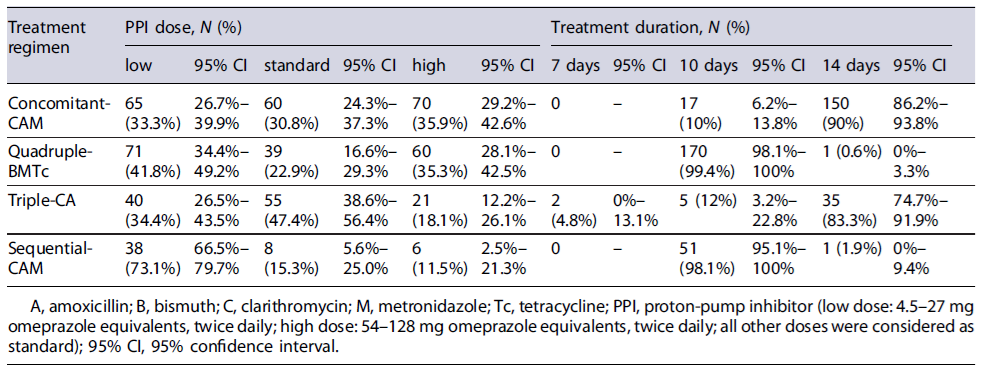
An eradication confirmatory test was performed in 649 patients, and the remaining were incomplete or lost to follow-up. The duration of treatment and the PPI dosage used in the most frequently prescribed first-line regimens are recorded in Table 3. There were no significant differences in effectiveness rates between the low-dose PPI versus standard/high-dose PPI, regardless of the length of the treatment, within quadruple-BMTc, concomitant-CAM, triple-CA, and sequential-CAM. Regarding the treatment duration, the majority (n = 320, 57%) of patients were treated with 14-day concomitant-CAM and 10-day quadruple-BMTc. Only 2 patients received a 7-day duration regimen. According to the analysis of all treated patients, those who received first-line treatments were predominantly treated with low-dose PPIs (n = 214, 38%).
Safety and Compliance
Two patients (0.4%) prescribed with triple-CA therapy discontinued the medication due to AEs (diarrhoea, nausea, and vomits). AEs were reported by 11% (n = 59), with dysgeusia (5%), nausea (4%), and diarrhoea (3%) being the most common. Among those who completed the eradication test, treatment compliance was achieved by 99% of the patients (643/649).
Rescue Therapy
From the 700 studied cases, 13% (n = 93) received a second-line empirical treatment, 5% (n = 36) received a third line, and 1% (n = 7) were prescribed with a fourth therapy line. Patients who underwent second-line treatment were mainly prescribed with triple therapy with amoxicillin and levofloxacin (n = 30, 32%), quadruple-BMTc (n = 25, 27%), and concomitant-CAM (n = 18, 19%). The mITT effectiveness of each of these second-line treatments was 60% (18/30), 91% (19/21), and 93% (14/15), respectively.
Temporal Trend Analysis
When evaluating the trends of H. pylori eradication success from 2013 to 2022 (Fig. 1), it was observed that the effectiveness, according to mITT, fluctuated along the years of the study. Figure 2 represents the evolution of the prescriptions between 2013 and 2022. The triple-CA therapy exhibited a declining trend after reaching its peak in 2019, with 0% prescriptions by 2022. A similar pattern was observed with sequential-CAM. Conversely, quadruple therapies, concomitant-CAM, and quadruple-BMTc were predominantly used since 2016, being the two most used regiments since 2017, with quadruple-BMTc being the top prescribed regimen since 2019 and reaching 76% of all prescriptions in 2022.
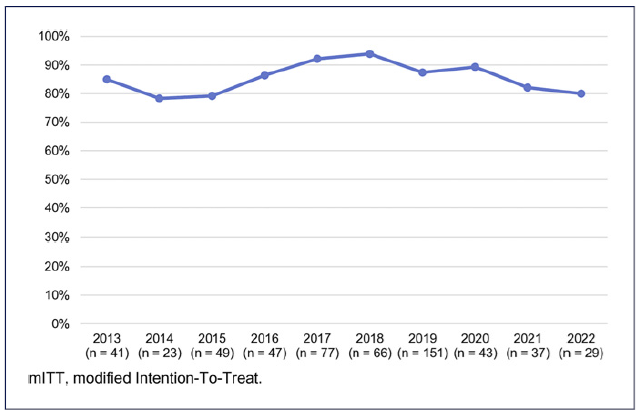
Fig. 1 Trends in H. pylori eradication effectiveness by mITT analysis during the years 2013-2022. mITT, modified intention-to-treat.
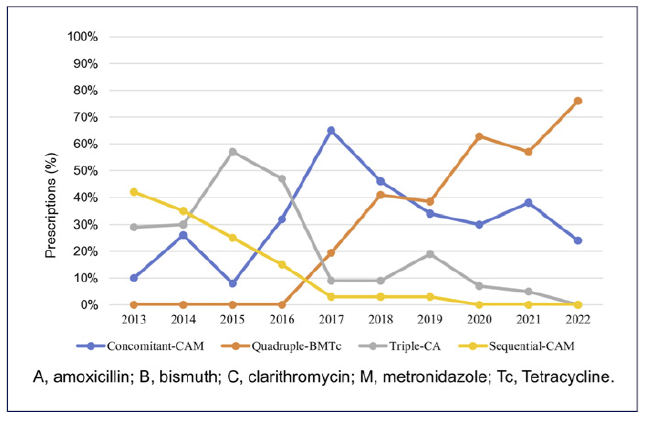
Fig. 2 Trends in the prescription of the four most frequently used first-line empirical treatments during the years 2013-2022. A, amoxicillin; B, bismuth; C, clarithromycin; L, levofloxacin; M, metronidazole; Tc, tetracycline.
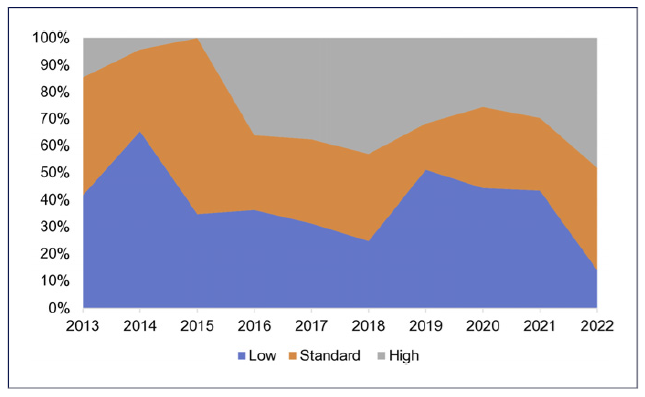
The trends in the prescription of PPI dosage are illustrated in Figure 3, reflecting a tendency towards an increase in the use of higher PPI doses and a decrease in standard and low doses between 2013 and 2022. In 2022, the rates for high versus standard versus low PPI doses were 48% versus 38% versus 14%, respectively. Also, trends regarding the length of prescription changed from mainly 7 days until the year 2016, where 10- and 14-day therapies became the preferred options. In 2022, these 2 lengths represented 86% of all prescribed regimens.
Discussion
In Portugal, the overall first-line treatment effectiveness in our cohort was reported as 87%, placing it within the category of borderline acceptability,according to the mITT analysis [12]. Similar results were obtained in the PP analysis, which can be attributed to the high rate of treatment compliance, due to the similar sample size. The absence of specificPortuguese guidelines that consider the local pattern of antibiotic resistance makes the treatment of H. pylori infection a persistent challenge.
Due to the unavailability of an optimal therapeutic regimen, there was a gradual evolution of prescriptions, involving mainly quadruple regimens, longer prescription durations, and higher PPI doses over time [17, 18]. In fact, in our study, it was observed that quadruple therapies achieved eradication rates of 91%, according to the mITT analysis, classified as good.
A study conducted by Nyssen et al. [13] with data from the Hp-EuReg, which included 21,533 treatment-naïve patients across 27 European countries distributed in five geographical regions, assessed the prescriptions and effectiveness trends of first-line empirical treatment over a 5-year period. The study reported that only quadruple therapies (with a minimum duration of 10 days) achieved nearly 90% eradication rates, with the quadruple-BMTc treatment proving highest results. Similarly, a Spanish Hp-EuReg sub-study concluded that the highest eradication rates were obtained with the same quadruple-BMTc (95%), the quadruple-BCA (91%), and the concomitant-CAM (90%), particularly when treatment was optimized (during 14 days and concomitantly with standard PPI doses, i.e., 40 mg omeprazole equivalent twice a day) [18]. Furthermore, in an Italian Hp-EuReg cohort study, it was observed that for the 10-day therapies, only quadruple-BMTc, concomitant-CAM, sequential-CAM, and 14-day concomitant-CAM reached an eradication rate ≥90% [19]. Our results in Portugal were in line with the aforementioned findings.
The use of quadruple-BMTc regimen represents the first-line option therapy in regions with high C resistance (>15%), given there is no resistance of H. pylori to B and minimal, if any, to Tc, which could eventually lead to the withdrawal of culture and susceptibility testing [20-22]. B as isolated medication is unavailable in Portugal, leading to the use of quadruple-BMTc therapy formulation as single capsule (marketed as Pylera®). This capsule is prescribed during 10 days. It has a 3-in-one capsule strategy (encompassing B, M, and Tc within one single capsule), intended to decrease the pill burden and enhance compliance [22]. This therapeutic option became commercially available in Portugal in March 2016, a detail that is evident in Figure 2 [20]. In our setting, should this capsule be locally unavailable, the recommended regimen is concomitant-CAM, which also achieved satisfactory results in our study. In line with our results, the finding was of a randomized trial reporting that, in fully compliant patients, the efficacy of concomitant-CAM therapy was 100% in Italy and 94.5%in Spain, with common mild side effects [22]. Another randomized controlled study compared the efficacy of 10-day concomitant-CAM versus 10-day quadruple-BMTc versus 14-day triple-CA as first-line treatments: while the 10-day quadruple-BMTc demonstrated superior efficacy (90%), the 10-day concomitant-CAM and 14-day triple-CA had lower eradication rates (86% and 84%, respectively) [23]. These findings suggest that a 10-day duration for concomitant-CAM therapy may be insufficient, and the recommendation to extend the duration to 14 days, as suggested in the Maastricht VI/Florence consensus report, should be considered [23]. In our cohort, the majority of concomitant-CAM was administered for a duration of 14 days, aligning with the current guidelines’ recommendations [1].
Triple-CA therapy remains the most used treatment option for the eradication of H. pylori infection in clinical practice in Europe [17, 24]. Nevertheless, only a limited number of regions remain below the threshold defined as low (<15%) C resistance, and a significant reduction in the proportion of eradication has been observed when this therapy is used [1, 9, 24]. It is possible to observe this change in our cohort, characterized by a decreasing trend in triple-CA therapy prescriptions during the studied time span. Despite the change in guidelines, which initially suggested that triple-CA therapy could be administered for 7 to 10-14 days, the recommendation transitioned in 2017 to a 14-day duration for this regimen [25, 26]. In our study, although seven (17%) patients underwent triple therapy either for 7 days or 10 days, the majority (83%) received the prescribed 14-day duration.
Sequential-CAM therapy demonstrates inadequate eradication rates in regions with dual resistance to M and C, a frequent scenario in Portugal [6, 22, 27]. However, it is important to highlight that, regarding sequential therapy, the sample size of patients prescribed with the referred therapy was inferior and the mITT analysis showed an effectiveness rate below 90%, which is in line with the current literature. Sequential-CAM therapy can be considered a complex regimen due to the required medication change midway through the treatment period; however, this did not impact compliance in our cohort [1]. Since it has shown inferior effectiveness compared to concomitant-CAM therapy, it is currently not recommended as first-line treatment in current European Consensus Guidelines [1, 24, 28]. Our results met the Maastricht VI/Florence consensus report, showing a decrease in the prescription of the afore-mentioned regimen [26].
When evaluating the prescription of the eradication schemes, we observed a marked heterogeneity, with 13 different therapeutic regimens prescribed as first-line treatments. The variability likely derives from the absence of Portuguese consensus guidelines during the study period. To ease the understanding of our data, our analysis focused on the four most prescribed therapeutical regimens covering 95% of the data cohort conferring sufficient representativeness of the sample. In 2019, there was an increase in the variety of prescriptions used, and we are unaware why. Also, although an association between the increased use of both concomitant-CAM and quadruple-BMTc treatment and a decreased H. pylori eradication treatment effectiveness was observed, with rates failing from 92% to 80%, we could not find an underlying cause for this association.
PPIs play a crucial role in H. pylori eradication treatment, as the effectiveness of antibiotics is optimized at higher gastric pH levels [16]. There is evidence suggesting that high-dose PPIs enhance eradication rates compared to standard doses, particularly in regions with high bacterial resistance [29]. A recent meta-analysis revealed that esomeprazole and rabeprazole exhibited superior eradication rates [30]. Latest VI Maastricht consensus guidelines state that high-dose PPI twice daily enhances the efficacy of triple therapy; however, in our cohort, low-dose PPI was twice the most frequent pre-scriptions as compared to higher PPI doses [1, 26]. We are unaware of the cause of non-adherence with the guidelines.
It is agreed that the best approach to have an effective H. pylori eradication is to succeed on the first-line treatment, to avoid retreating and retesting [31]. Since compliance is one of the strongest independent factors associated with effectiveness, it is of utmost importance to achieve it [13, 22]. In our cohort, treatment compliance was reported in 99% of the patients, which was excellent. Another important factor together with compliance is reducing the impact of bacterial antibiotic resistance, an envisioned goal for an optimized therapeutical approach. However, currently there is no sufficient evidence to advocate the generalized use of susceptibility-guided therapy for H. pylori treatment in real clinical practice, given continuous monitoring of the current resistance prevalence would be essential as well as challenging, to assess which treatment would be more effectively tailored to meet specific cure needs [32, 33].
Among the limitations of this study, the fact that the Hp-EuReg is not a randomized, controlled trial might affect the interpretation of the results. And so, in current observational study, there is no information regarding the rationale behind the physicians’ prescriptions. Another drawback is that we had few centres participating, most of the cases coming mainly from two centres, and all being tertiary departments; so, only gastroenterologists recruited patients for inclusion, without representation of primary care facilities. During the data analysis, the presence of collinearity within our cohort prevented us from conducting a multi-variate analysis. The univariate analysis revealed no sig-nificant differences in eradication rates between the various treatment groups. As a result, our findings are based on the descriptive data from our cohort.
The strengths of our study include its prospective and real clinical practice nature. Our study evaluated the patterns of therapeutic regimens prescribed over 10 years of clinical practice, covering a wide range of years, which offers the scientific community a comprehensive perspective on the evolution of local anti-H. pylori eradication strategies. This provides the Portuguese literature with significant insights into H. pylori treatment management, with robust conclusions that can be readily applied in clinical practice.
To conclude, the Portuguese Hp-EuReg sub-study found that concomitant-CAM and quadruple-BMTc therapies
achieved optimal (>90%) effectiveness, aligning with those therapies achieving optimal effectiveness in other Southern European countries, also characterized by similar high antibiotic resistance prevalence patterns. There was a verified discontinuation of triple-CA therapy, with preferred eradication regimens evolving to mainly quadruple regimens, longer treatment durations, and higher PPI doses over time, which was in accordance with the international guidelines. Periodic studies like this are valuable for gathering information on adherence to guidelines and assessing the effectiveness of the current therapeutical regimens implemented in Portugal as well as identifying the room for improvement in our setting. Looking ahead, future research should focus on tailoring therapies based on local data to enhance treatment outcomes and reduce the emergence of antibiotic resistance.