Introduction
Tuberculosis (TB) results from Mycobacterium tuberculosisinfection, which infects almost a quarter of the world’s population and remains an important cause of ill health and mortality worldwide [1]. Over 90% of infected individuals remain asymptomatic and are not infectious due to the persistent immune response to M. tuberculosis that persists within the human host as latent TB infection (LTBI). People with LTBI are at risk of developing active TB disease and becoming infectious during their lifetime, leading to death if left untreated [2]. Specific groups of people have a high risk of developing TB, including contacts of TB cases [3-5], HIV-positive people [6], healthcare workers (HCW) [6, 7], immigrants from countries with a high TB burden [3], immunocompromised patients [6], prisoners, homeless people, and illicit drug users [8].
The prophylaxis of individuals with LTBI is a strategy to control active TB, but there are no accurate methods to identify these individuals [1]. Diagnosis of LTBI relies on the measurement of host immune responses to M. tuberculosis antigens which are based on a specific immune response against the bacillus, by using the tuberculin skin test (TST) or interferon-gamma (IFN-γ) release assays (IGRA) [2, 9-11]. The TST measures the delayed-type hypersensitivity response to intradermal injection of purified protein derivative, a crude mixture of several mycobacterial antigens which are common to M. tuberculosis, M. bovis BCG, and non-tuberculous mycobacteria (NTM) [10]. In turn, IGRA is an in vitro blood test, detecting the release of IFN-γ by circulating T-lymphocytes in response to epitopes from specific M. tuberculosis complex-associated antigens, namely ESAT-6 and CFP-10 [2, 11]. The TST is a simple and cost-effective test, but it needs a follow-up visit for result interpretation [10, 11], while IGRA requires blood samples and a laboratory to process them quickly after collection [11].
There is a body of research on comparing the performance of TST and IGRA in the general population [12] and in specific population subgroups such as children, immunocompromised people, those from countries with high TB rates [13], contacts of active TB cases [4, 14, 15], homeless people, refugees, HIV-positive cases [16], and HCW [14, 17-22]. There are no consistent results across studies and such variability could be explained by differences in demographic characteristics [16, 20], the community TB burden [4, 13], the immune status [13], or the prevalence of the BCG vaccination [16, 20].
Although the TB incidence rate in Portugal has been decreasing, it remains one of the highest TB rates in Europe [23], demanding effective identification and treatment of individuals with LTBI. As far as we know, the performance of TST and IGRA for diagnosing LTBI in the Portuguese population was limited to the screen of HCW [21, 22]. Thus, we aimed to determine the proportion of LTBI by using the TST and the IGRA test and to assess the risk factors related with discordant results between tests across several risk groups advised for screening in Northeast Portugal.
Materials and Methods
This study was carried out in a public health unit in the Northeast Portugal. Data were collected from the database of patients with suspected LTBI and advised for screening. In our setting it is mandatory to fill in a questionnaire asking for relevant information before patients undergo screening tests for LTBI. The questionnaire asks for the following information: age, gender, BCG vaccination status, HIV/SIDA diagnosis, and the reason for the suspected LTBI. All 377 patients screened between January 2014 and December 2015 who underwent both TST and IGRA were considered eligible participants. After excluding 3 (0.8%) patients with indeterminate IGRA and 7 (1.9%) patients due to a diagnosis of active TB, 367 participants were included in the present analysis.
TST and IGRA
Blood was collected for the IGRA and analyzed with the QuantiFERON Gold In-Tube assay (Cellestis/Qiagen, Dusseldorf, Germany). The IGRA method quantifies the IFN-γ released after the incubation of blood with a cocktail of peptides derived from ESAT-6, CFP-10, and TB7.7 (Rv2654c) antigens [24]. The IGRA was considered positive if the level of IFN-γ was 0.35 IU/mL or more [24].
The TST was performed by using an intradermal injection of 5 U of tuberculin (Pasteur Institute, Paris, France) on the volar side of the forearm. The transverse diameter of induration was read 72 h later [25]. Following recent guidelines, the TST was considered positive if the diameter of induration had 5 mm or more among children aged less than 5 years old, immunocompromised people, and those with a diagnosis of HIV/SIDA [26].
Potential Predictors
The following variables were considered potential predictors of TST or IGRA positivity and were retrieved from the database: gender, age, BCG immunization, HIV status and risk group for TB (contacts of active TB, HCW, inmates, immunodepression status, and other circumstances).
Statistical Analysis
Participants were classified as LTBI positive or LTBI negative for each test (TST and IGRA) and were categorized into four groups: negative for both tests (TST-/IGRA-), positive for both tests (TST+/IGRA+), discordant with positive TST (TST+/IGRA-), and discordant with positive IGRA (TST-/IGRA+). The proportion of positive TST (≥5 mm for children aged less than 5 years, immunocompromised people, and those with a diagnosis of HIV/SIDA or ≥10 mm for other people) or positive IGRA (≥0.35 IU/mL), concordant positive, concordant negative, and discordant tests were computed. Bivariate analysis was performed to compare groups according to the TST and IGRA results. Multivariate logistic regression analysis was performed to evaluate the association between a positive TST, a positive IGRA, or a discordant result as dependent variables, and the age, gender, HIV status, BCG immunization status, and the risk group of the patients as potential predictors of LTBI proportion. Estimates of odds ratio (OR) adjusted for all these potential predictors and the respective 95% confidence interval (95% CI) were obtained. Analyses were performed using the SPSS statistical software for Windows (SPSS version 26.0). The general significance level was set to 0.05.
Results
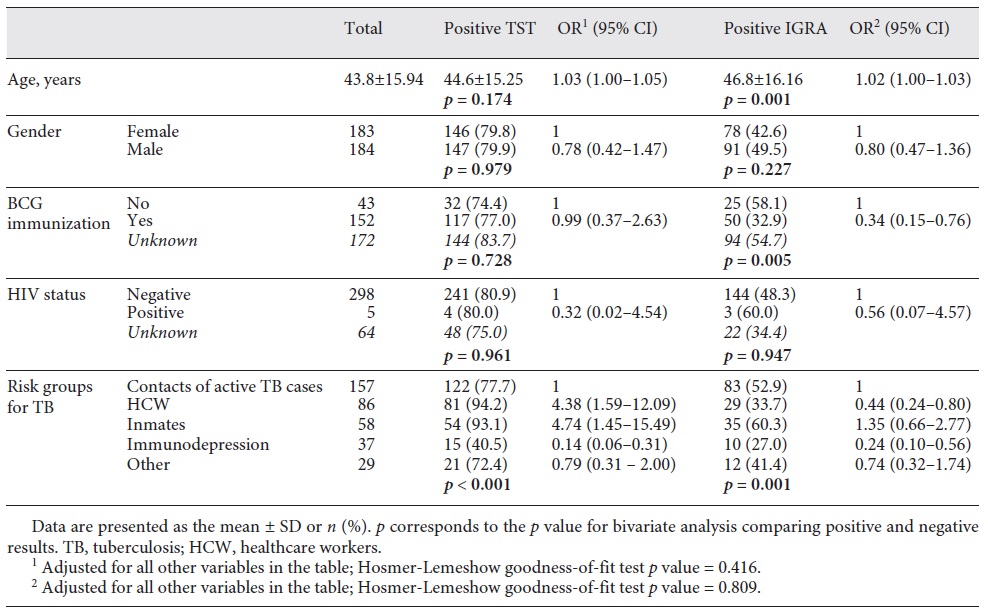
A total of 367 individuals were included in this analysis; 42.7% (n = 157) were contacts of active TB cases, 23.4% (n = 86) were HCW, 15.8% (n = 58) were inmates, 10.1% (n = 37) were immunosuppressed people, and 7.9% (n = 29) corresponded to other risk groups. Out of all of the participants, 79.8% (n = 293) had a positive TST and 46.0% (n = 169) of them had a positive IGRA.
Table 1 presents the proportion of positive TST or positive IGRA. According to the bivariate analysis, the proportion of positive TST was significantly different across risk groups for TB (p < 0.001), varying from 40.5% (immunocompromised people) to 94.2% (HCW). The proportion of IGRA positivity was significantly different between groups by BCG immunization (p = 0.005), varying from 32.9% (vaccinated) to 58.1% (non-vaccinated), and across risk groups for TB (p = 0.001), varying between 27.0% (immunocompromised people) and 60.3% (inmates). After adjustment for all other variables, older people were more likely to present TST positivity (OR 1.03, 95% CI 1.01-1.05) or IGRA positivity (OR 1.02, 95% CI 1.00-1.03). Compared with TB contacts, the odds of TST positivity was higher among HCW and inmates (OR 4.38, 95% CI 1.59-12.09 and OR 4.74, 95% CI 1.45-15.49, respectively) but lower among immunocompromised subjects (OR 0.14, 95% CI 0.06-0.31). The odds of IGRA positivity were lower among BCG-vaccinated people (OR 0.34, 95% CI 0.15-0.76) compared with non-vaccinated people. When compared with TB contacts, HCW and immunocompromised people presented lower odds of a positive IGRA (OR 0.44, 95% CI 0.24-0.80 and OR 0.24, 95% CI 0.10-0.56, respectively).
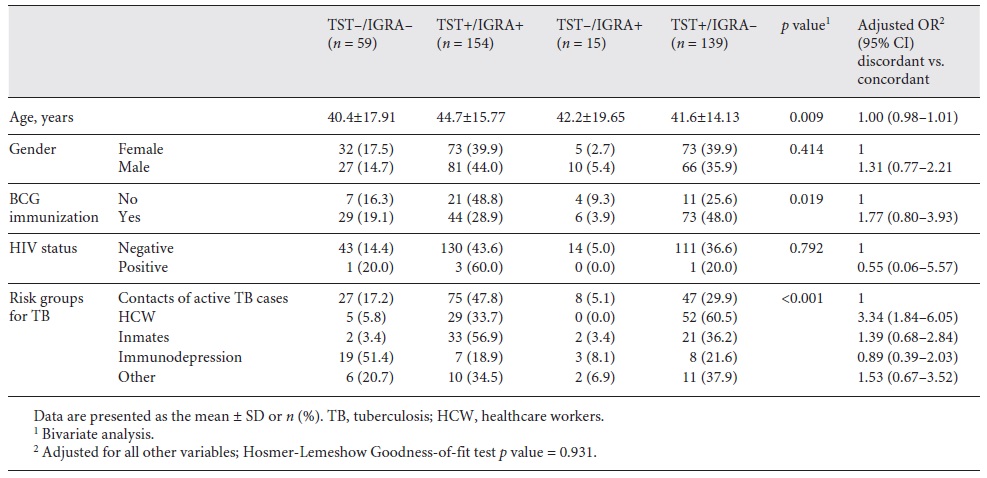
Table 2 shows the proportion of discordant and concordant results by groups according to the potential predictors. There were 42.0% (n = 154) TST+/IGRA+, 16.1% (n = 59) TST-/IGRA-, 4.1% (n = 15) TST-/IGRA+, and 37.9% (n = 139) TST+/IGRA-. According to the bivariate analysis, some variables presented a significant association with discordant results, namely age (p = 0.009), BCG immunization (p = 0.019), and the risk groups for TB (p < 0.001). However, after adjustment for all other variables, only risk groups for TB were related with a discordant result; compared with TB contacts, HCW had 3-fold higher odds of a discordant result (OR 3.34, 95% CI 1.84-6.05).
Discussion
This is the first study conducted in Northeast Portugal on the proportion of LTBI, for which purpose we used TST and IGRA. According to our results, the proportion of LTBI estimated by IGRA and TST was 46 and 80%, respectively. Among HCW, the proportion of positive IGRA was 34%, similar to data reported in previous research conducted in Portugal [21]. However, according to a recent systematic review, the prevalence of LTBI among HCW by using IGRA in low-incidence countries was only 16% [27]. Also, the proportion of LTBI we found among TB contacts and inmates was higher than reported in other European settings [4, 14, 28, 29]. Our findings probably reflect the TB burden in Portugal, a country with one of the highest incidence rates (20.5 new cases and relapses of TB per 100,000 population) and with one of the lowest TB treatment success rates (37.3%) among European countries in 2018 [23].
Previous research has reported the association between positive results with TST or IGRA and several predictors of TB [5, 12, 15, 17-20], but there are no consistent results across studies. We found an association between age and positive results with both tests, with older people presenting a higher risk of a positive test, in accordance with some previous studies [12, 15]. However, in other studies, age was related only with IGRA results [14, 18] or no association existed between age and positivity, either with TST or IGRA [5, 19, 20].
We found no association between BCG immunization and TST positivity. Interestingly, IGRA positivity was less likely among immunized people, in accordance with previous research suggesting the relevance of the IGRA screening test in LTBI evaluation [12, 19]. The higher specificity of IGRA to detect M. tuberculosis infection [29] and the protective effect of BCG vaccination on M. tuberculosis infection [12] could explain our results.
Despite the lack of a gold standard to diagnose LTBI, we compared IGRA with TST results for estimate the proportion of LTBI among people with suspected LTBI and advised for screening. Our findings showed a low rate of TST-/IGRA- (16.1%), while the rate of TST+/IGRA+ reached 42.0%. Previous research enrolling TB contacts and HCW [14, 17, 19] reported a lower rate of TST+/IGRA+ and higher rate of TST-/IGRA- than we found. In some settings the diagnosis of LTBI is only considered when both tests are positive [3]; therefore, our results suggest a high risk of LTBI among people advised for the screening in our setting, demanding preventive strategies against TB.
The proportion of LTBI we found was higher when using TST than when using IGRA, which leads to a higher proportion of TST+/IGRA- among discordant results, and these findings are in accordance with previous research [14, 17, 19, 20]. However, our rate of discordant results (41.9%) was higher, particularly among HCW (61%), than reported in previous research [14, 17, 19]. According to our results, in comparison with TB contacts, HCW presented a higher proportion of TST positivity but lower proportion of IGRA positivity. It has been reported that IGRAs provide higher specificity than the TST [30]. The TST is more sensitive than IGRA [2, 11, 30] as a positive TST may be due to BCG vaccination or exposure to environmental NTM [2, 11]. According to our results, BCG vaccination did not explain discordant results after adjusting for the other variables, indicating the impact of other factors, rather than BCG vaccination, in explaining discordant results. Indeed, only HCW remains a predictor of discordant results, and the discordant results in this group are TST+/IGRA-. Repeated TST as a surveillance strategy among HCW could boost the TST reaction among those with a previous M. tuberculosis or NTM infection [31, 32], similar to what has been observed among BCG-vaccinated people [32]. It is not clear whether these results could be explained by the exposure to NTM [2], as NTM infections appear responsible for positive TSTs among HCW [31].
Based on WHO recommendations, either TST or IGRA can be used to evaluated LTBI, because no strong evidence suggests that one test should be preferred over the other for predicting progression to active TB disease [8]. However, the TST and IGRA results we observed across risk groups suggest the need for caution in reading the TST, as it seems be affected by other factors rather than recent exposure to M. tuberculosis.
The current study gives insights concerning the proportion of LTBI in several risk groups for TB by including all individuals with suspected LTBI in a specific community in Northeast Portugal who were advised for the screening. Nevertheless, there are some limitations. As we used the data from a single public health unit, we should be cautious in generalizing the findings. Furthermore, we missed some details. We have no information about the number of years in the healthcare profession, level of occupational risk or job category, and the time of exposure to M. tuberculosis, which have been reported to be associated with LTBI [4, 17, 33].
Conclusion
The proportion of LTBI estimated by TST and IGRA is high among people advised for LTBI screening in Northeast Portugal, highlighting the need for strategies to control TB. The interpretation of TST results requires caution, particularly among HCW, due to a higher proportion of TST+/IGRA-, suggesting the impact of the booster reaction to previous M. tuberculosis exposure. Further research is needed by including more detailed information about participants.
Acknowledgements
The authors thank the Unidade Local de Saúde do Nordeste for allowing access to the data.
Statement of Ethics
The authors declare that they complied with ethics guidelines. The study protocol was approved by the Ethical Committe of the Unidade Local de Saúde do Nordeste (No. 28/2020).
Author Contributions
A.L.F.A. conceived this study and collaborated in the data collection; B.M.M.P. collected the data; C.M.T. carried out the statistical analysis of the data. A.J.N. supervised all aspects of this work. All of the authors contributed to the interpretation of the results, writing the article, and proof reading of the manuscript.