Introduction
Coronavirus disease-2019 (COVID-19) was first reported in December 2019 in Wuhan, China, and rapidly spread worldwide causing the second pandemic of this century, after the 2009 H1N1 pandemic [1, 2]. Worldwide data points to rates higher than 90% of asymptomatic children or children with mild to moderate disease symptoms, and the death rate is lower in children when compared to adults and elderly [1, 3]. It is still unknown if there are long-term effects of SARS-CoV-2 infection, even in milder cases.
In Portugal, the first pediatric case was reported on March 7, 2020, and by the end of May, a total of 1,771 cases between 0 and 19 years of age were reported [4]. Between April and May 2020, cases below 19 years old accounted for 6.2% of total cases, and there were no casualties in this age group [4].
Due to little and inconsistent knowledge about COVID-19 in children, national and international guidelines were not easily adapted to departments of pediatrics, leading to an increased constraint on healthcare systems and their workers.
Protecting healthcare workers (HCW) from contracting SARS-CoV-2 should be a main priority to protect and keep healthcare systems working. Understanding the modes of transmission is essential to adopt the right preventive measures. SARS-CoV-2 is primarily transmitted person-to-person through respiratory droplets containing the virus and through close contact [3, 5]. Airborne transmission is possible when aerosol-generating procedures are performed, but it is not yet known if in their absence this transmission can still happen [5]. Knowledge of these data and the uncertainty of what is yet to be known generates inconsistencies regarding personal protective equipment (PPE) recommendations [5]. Control measures start long before a suspected patient reaches an HCW. Source control, triage systems to identify suspected patients, and course of actions to minimize contact with COVID-19 patients are the general lines of implemented measures in healthcare systems worldwide [5]. Correct PPE use is an important line of defense for HCW in the observation and treatment of suspected COVID-19 patients [5].
Our study aims to characterize how Portuguese National Health System Departments of Pediatrics have adapted to the pandemic and what measures were taken to best protect their HCW during the beginning of the pandemic, in April and May 2020.
Materials and methods
Study sample, period, and design
To evaluate how national departments of pediatrics adapted to the COVID-19 pandemic between April and May 2020, a descriptive study was implemented using an unvalidated, anonymous questionnaire based on COVID-19 national guidelines and orientations. It consisted of 47 questions, of which 19 were dichotomous questions, 17 multiple-choice questions, 10 text numeric questions, and one single row text questions. The questionnaire was sent by e-mail to all National Health System Departments of Pediatrics directors. Data regarding neonatology units were excluded.
Data collection and analysis
The questionnaire assessed departments’ demographic characteristics, such as region, hospital’s typology, and possessing an intensive care unit. Departments of pediatrics directors were then asked about emergency ward (EW) re-organization (physical space and HCW teams), the PPE used in the EW, appointments, and how many HCW were tested and were found to be positive. Criteria and methods used for testing were asked for.
All answered questionnaires were included in the analysis if submitted until June 30, 2020, and were excluded if they had more than 50% of unanswered questions.
All data are presented as means and standard deviations, or medians and 25th-75th percentiles, according to the variables’ distribution. The descriptive statistic was performed using IBM SPSS® Statistics for Windows, version 26.0.
Ethics Questionnaire
The questionnaire used in this study was developed based on national directives and guidelines regarding COVID-19 infection. Participants were informed about the study aims through e-mail. Participation was voluntary and anonymous. The survey was sent by e-mail (a link for the anonymous questionnaire) to all Portuguese departments of pediatrics directors, and consent was assumed when an answered questionnaire was obtained.
Results
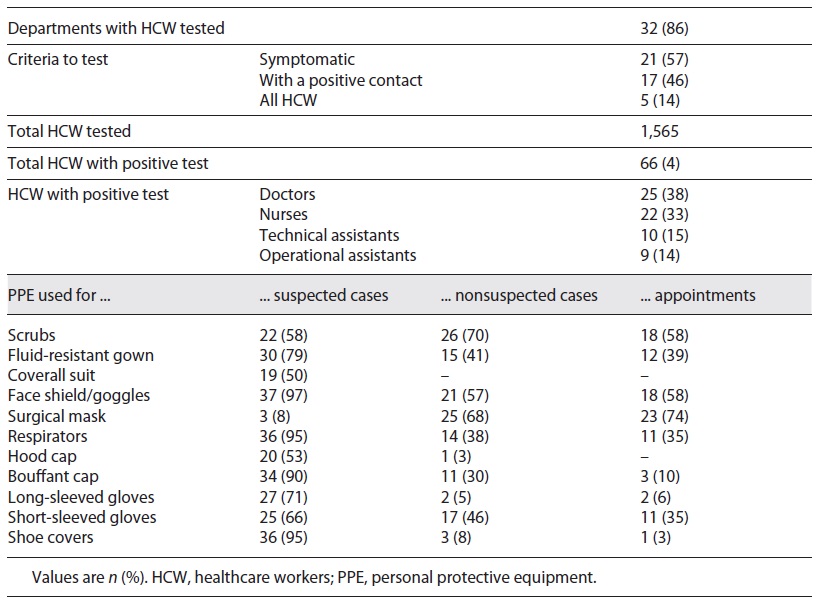
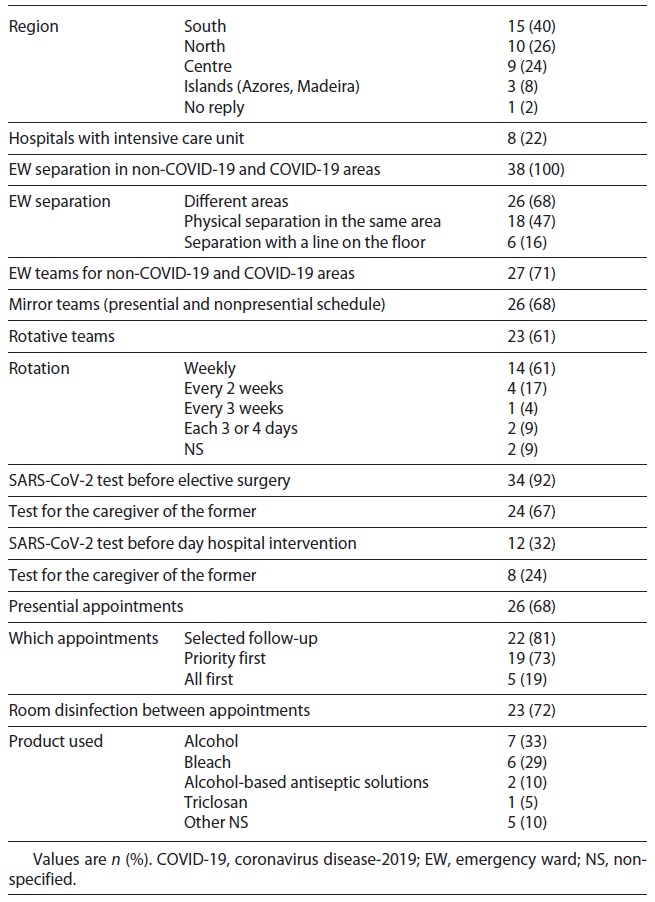
In this study, we included 38 questionnaires, representing 93% of the National Health System Departments of Pediatrics. In the studied months, EWs were divided into non-COVID-19 and COVID-19 areas in all hospitals: 68% rearranged the space in 2 separated areas, 47% created a physical division of the same space, and 16% drew a line on the floor. Ten departments (26%) changed the way of division during the study period, 4 between the physical barrier and line on the floor (11%), the other 4 between 2 separated areas and a physical barrier (11%), and 2 changed among the 3 options (5%). Medical staff constituted different teams responsible for each area in 71% of the hospitals, in which 68% had a presential and nonpresential schedule (mirror teams) and 61% had rotative teams. All children submitted to an elective surgery were tested previously, and in 32% of the hospitals, children checked at outpatient clinic care were also tested. Presential appointments were carried out in 68% of the surveyed departments, mainly including selected follow-up and priority-first appointments (Table 1).
Table 1 Characterization of departments of pediatrics in Portuguese National Health System hospitals in response to the COVID-19 pandemic
In 86% (n = 32) of the hospitals, HCW were tested, 57% for presenting symptoms and 46% for being contacts of confirmed cases. In none of the departments, tests were performed routinely in all HCW. Among a total of 1,565 professionals, 66 were positive for SARS-CoV-2 (representing 4% of the reported HCW tested), 25 doctors and 22 nurses.
Table 2 shows the PPE used for different contexts: face shield/goggles were used by professionals in 97% of the hospitals and respirators in 95% for the observation of suspected cases. Long-sleeved (71%) and short-sleeved gloves (66%) were also used for suspect patients. Scrubs and surgical masks were used in 70% of departments of pediatrics to exam nonsuspect cases in the EW. PPE used for children’s appointments were similar to the ones used for the observation of nonsuspected cases in the EW: surgical mask in 74 and 68%, and face shield/goggles in 58 and 57% of hospitals, respectively. PPE used for suspected cases was the same for all ages in all hospitals; however, for the observation of nonsuspected children, there were differences in PPE choice according to age in 8% of the hospitals.
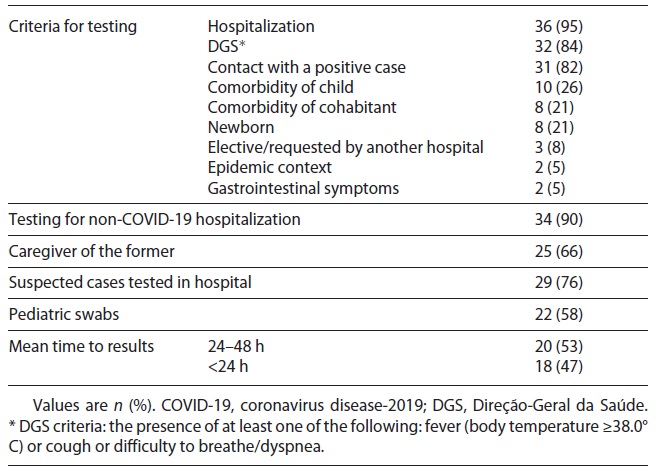
In Table 3, we present the criteria used for testing, with 84% of hospitals applying Direção-Geral da Saúde (DGS) criteria (fever ≥38.0°C or cough or difficulty to breathe/dyspnea); 82% tested if the children had had contact with a confirmed positive case. The most frequently used criterion for testing suspected children was hospitalization in 95%. If nonsuspected children needed hospitalization, 90% of hospitals tested them and 66% tested their caregivers. Seventy-six percent tested children in the hospital, regardless of age, and the other hospitals referred them to community COVID-19-dedicated areas to perform the test.
Pediatric swabs were used in 58% of the hospitals and the results were available in less than 24 h in 47%. Ten hospitals (27%) transferred 69 positive children to another department of pediatrics: 30% were 1-5 years old, 23% were 10-18 years old, and 7% were newborns.
Discussion
The value and importance of infection prevention and control measures were proved during the SARS-CoV-2 pandemic. The need to protect HCW to prevent health services from collapse led to reorganization and exceptional procedures in departments of pediatrics.
By the end of March 2020, the DGS advised health institutions to take measures to minimize the spread of infection and instructions for use of PPE [6]. Those included reductions of presential appointments, setup of circuits to divide COVID-19 suspects from non-COVID-19 patients, and restraining the number of HCW in contact with SARS-CoV-2-confirmed patients [6]. All national departments of pediatrics divided the EW into non-COVID-19 and COVID-19 areas, but in different ways; about two-thirds (68%) of hospitals used different areas, around half of them (47%) divided the same space with a physical barrier and 16% with a line on the floor, while 10 changed the mode of division during the study time. As we understand it, these different adaptations were due to space and material available in each department. Unfortunately, no record of which adaptation was done first was made. Future studies should examine how patients were divided before and during EW triage and how waiting rooms were organized. The majority of those who responded also created non-COVID-19 and COVID-19 teams (71%) and mirror teams (68%). Besides diminishing contact between HCW and consequently constraining infection, these decisions also allowed the protection of risk groups among HCW.
To minimize HCWs’ risk of exposure to the new coronavirus, PPE must be used as recommended and correct donning and doffing is essential to prevent SARS-CoV-2 infection. Based on the currently known modes of transmission of SARS-CoV-2, national and international authorities issued PPE recommendations when in contact with confirmed or suspected COVID-19 patients [5, 6]. Surgical masks and eye protection are recommended for droplet safety [5]. If aerosol generation procedures are made, respirators must be used instead of surgical masks [5]. On the other hand, contact precaution is based on the use of water-resistant gowns and gloves [5]. Several international guidelines and reviews state that fluid-resistant gowns, gloves, respirators, and goggles or face shields are the adequate PPE to use when suspected COVID-19 patients are observed in the EW [7]. DGS guidelines during the study period added the use of shoe covers and hood caps and stated that bouffant caps should only be used when high-risk procedures (tracheal intubation, tracheostomy, and bronchoscopy) are performed [6]. The majority of those who responded confirmed that in their department HCW used respirators, face shield or goggles, shoe covers, bouffant caps, and fluid-resistant gowns when in contact with suspected COVID-19 cases (Table 3). In just one department the use of gloves (long- or short-sleeved) was not reported as part of PPE used in suspected COVID-19 cases. With these results, we can assume that most departments protected their HCW from droplet and contact transmission but with different components. Regarding nonsuspected cases, the DGS advised using a surgical mask, and apron and gloves if organic fluid contact was predicted [6]. Except for scrubs, the surgical mask was the most frequently used component (68%), but others were used in different percentages and without concordance among departments. The lack of certainty concerning the mode of transmission of SARS-CoV-2 and the fear HCW have of becoming infected and spreading the disease through close relatives are some of the possible explanations of the apparent excessive use of PPE in these settings. DGS recommendations do not differentiate between the protections to be used by HCW in contact with suspected children irrespective of the age group. We suggest that further research should be undertaken to establish if any difference exists in national departments.
DGS guidelines regarding suspected HCW state that SARS-CoV-2 testing should be performed if an exposed HCW develops symptoms [8]. One half of those surveyed reported that HCW were tested because they were symptomatic (57%) or had contact with SARS-CoV-2-positive cases (46%). Regular testing of HCW was not performed. The option to do so could have identified infected cases at an early stage, avoiding possible in-hospital outbreaks. It was not asked in what circumstances contact with a SARS-CoV-2-positive case occurred (in a personal or professional context) or if in the professional context PPE was used correctly. We suggest that further research should be undertaken regarding nosocomial infection and in-hospital outbreaks, so that a better understanding regarding the protective measures taken can be reached and they can be reduced.
Since the COVID-19 pandemic declaration, Portuguese diagnostic criteria have been regularly revised, although our health authorities do not distinguish between criteria for children and adults. At first, epidemiologic and clinical criteria were used to consider a suspected case and testing was only done after a call to a physician support line (Linha de Apoio ao Médico) to validate the case as a suspect [9]. By March 23, 2020, the DGS stated that anyone with cough, fever, and/or dyspnea was considered suspect and should be tested [10]. Hospitalization was the most frequently reported criterion for testing (95% with symptoms of COVID-19 and 90% non-COVID-19 patients). Most departments also tested according to DGS criteria (84%) and when there was contact with a positive case (82%).
There are some singularities in pediatrics, namely the need for caregiver support during a child’s hospitalization. This raised questions about the need to test caregivers. Although most departments tested children without COVID-19 symptoms when hospitalized, only two-thirds tested their caregivers. A consensual approach regarding testing children before elective surgery and non-COVID-19 hospital admissions is of note, but less than half performed the SARS-CoV-2 test before outpatient clinic care. Our results also show that in the study period, most departments’ test results were available after 24 h and almost half did not have pediatric swabs - two aspects needing improvement, which we believe have already been overcome in the meantime.
More than two-thirds of departments (68%) maintained presential appointments, most of all selected follow-up and/or priority-first appointments. Telephone consultations could have been a form used to maintain assistance activity in most departments, a way not only to adhere to children’s and adolescent’s follow-up, but also to prevent SARS-CoV-2 spread [6, 11]. Regarding PPE use in appointments, the DGS states that nonsuspected patients should be observed with surgical masks and, if fluid contact is expected, gloves and a fluid-resistant apron [6]. Even though those interviewed reported high use of surgical masks (74%) or respirators (35%), the use of other PPE reported was not in line with these guidelines, with more than half using face shields or goggles.
The main limitations of this study come from its being an online questionnaire. Besides the possible different ways of analyzing and interpreting the questions, we also had some questions that were left unanswered and did not permit further analysis of other topics. Our questions were mostly dichotomous and multiple-answer and, after analyzing data, some of them deserved a fuller answer.
National Health System Departments of Pediatrics faced the pandemic differently and measures taken in the EW were the most consistent. When setting COVID-19 and non-COVID-19 circuits in the EW, ideally these should be done using different areas to minimize contact between the suspected and nonsuspected cases. The lack of material and infrastructure to create adequate division is an obstacle, reflected in 26% of departments who changed the way of division during the 2 months of the study. It would be of value to create plans of action for future infectious diseases like COVID-19, where the need for space and HCW division should be included, showing what we are learning from this pandemic.
We advocate the conception of national Pediatric guidelines for the approach of suspected children with the reminder that new evidence is being released constantly and the update of guidelines should also be a priority. Regarding PPE, adequate use was reported in all settings and even with more components than those described as needed by national and international guidelines. Resources should be secured to protect HCW.
Acknowledgement
The authors thank all the departments who answered the questionnaire, allowing this work to be written.
Statement of Ethics
The authors declare that they complied with ethical principles according to the Declaration of Helsinki. Any opinions, findings, conclusions, or recommendations expressed in this article are those of the authors.
Author Contributions
B.S.V. did the bibliographical search, analysis of the results, and drafting of the article. M.L.C. carried out the statistical analysis of the data and drafting of the article. J.A. collaborated with the analysis and interpretation of the results and critical review of the content of the article. B.X. supervised all aspects of this work. All authors contributed to the study design and proofreading of the manuscript.