Introduction
The most accessible information source that patients receive about medicines is the patient information leaflet (PIL), which contains information about the appropriate use of each medicine. In the European Union, all medicinal products available on the community market must be accompanied by labelling (printed materials that accompany drugs in their packages) and the corresponding PIL, which must include a set of comprehensible information enabling the use of the medicinal product safely and appropriately, in addition to the information that might be provided by health professionals 1,2.
The European legislation for the labelling and packaging of leaflets of medicinal products for human use, published in 2009, establishes the requirements for PILs and it is defined that its elaboration must be performed in accordance with the summary of product characteristics. A set of parameters are required in the preparation of PILs which are described in the referred legislation. It includes type size and font, design and layout of the information, headings, print colour, syntax, style, paper, and use of symbols and pictograms 2.
According to current European legislation, PILs preparation must include a consultation with target patient groups (“user consultation”) to demonstrate the readability and usefulness of these documents to the consumers 2. In addition, user testing must be carried out to assess the readability of the PILs using a group of selected subjects.
The user testing results, required for European PILs, must be presented to the regulatory authority (INFARMED, in Portugal) including the following mandatory items: product description, consultation or test details (method used, explanation of the choice of population consulted, language(s) tested), questionnaire (including instructions and observation forms), original and revised package leaflets, summary and discussion of results (subjects’ answers, problems identified, and revisions made to relevant package leaflet section), and conclusion 2. Despite the existing European regulations for PILs, usability is not guaranteed, and some issues have been identified giving rise to the necessity of improving the content and layout of the leaflet within the regulations 3. Although the main goal of including PILs in the medicinal products packages is to provide information to users, the amount of information available in PILs does not seem to be meeting the needs of patients, particularly in the case of older or illiterate people 4,5.
The readability assessment of a document can be performed using several methodologies, such as the Gunning Fog Index and the Flesch Reading Ease score. The Flesch Reading Ease score provides a numeric representation of reading difficulty ranging from 0 (extremely difficult) to 100 (extremely easy). It is expected that most of the population can understand the written material and have an index of around 60-70 6.
The Portuguese population is an aged population and, according to the projections, between 2012 and 2060, the ageing index should increase from 131 to 307 older adults per 100 young people in a central scenario (not too optimistic nor pessimistic). In that same period and scenario, the potential sustainability index should change from 340 to 149 working people per 100 older adults. The average life expectancy should be 84.21 years, for males, and 89.88 years for women, which means that an increase in the older population is to be expected 7.
The increase in average life expectancy of the population leads to greater use of pharmacological treatments and, consequently, to an increase in their side effects. These facts make the older population more vulnerable and more in need of means that allow them to understand and contribute to a responsible use of the medicine 8. Physiological changes resulting from ageing have an impact on the pharmacokinetics and pharmacodynamics of drugs, often leading to the need for adjustments, namely in doses or drug selection by this population 9. Despite the mandatory presence of PIL within the medicines’ packages, it is important that its content is tailored to the several profiles of readers, particularly older patients 10. The main goal of this study was to assess if PILs content of several medicines is appropriate to older patients.
Materials and Methods
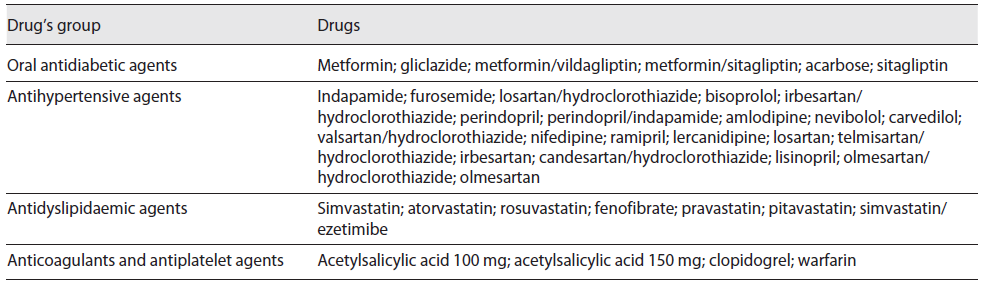
The selection of the PILs to be analysed was made based on the following inclusion criteria: (1) belong to the top 100 active substances with highest number of packages consumed in Portugal according to a national report of INFARMED, during 2017 11; (2) belong to the pharmacotherapeutic groups more used by patients with cardiometabolic chronic diseases (diabetes mellitus, hypertension, and dyslipidaemia), which are very common in the older population and also due to its association to the increase of cardiovascular events which is the main cause of death in the Portuguese population 12; (3) to be consumed in solid oral forms; (4) PIL included in the largest package size (assuming is the most used by patients with chronic diseases); (5) PIL included in the package with a dose of active substance corresponding to the strength of the defined daily dose, according to the ATC/ defined daily dose index 13. The active substances (drugs) included are listed in Table 1.
Table 1 List of drugs included for the analysis of their respective PILs divided by groups according to the corresponding pharmacotherapeutic groups
After applying the inclusion criteria mentioned, a subsequent selection was made to narrow the list. Considering that medicines prices applied in the National Health System are reviewed on a trimestral basis, the correspondent INFARMED deliberation was used, and, for each drug, the most expensive brand medicine and the less expensive generic medicine were identified and selected, when applicable. Only the brand name medicine was included for cases where no generic medicine was available.
PILs analysis included legibility, readability, content (technical information including dosage instructions, precautions, and adverse effects), and availability of direct or indirect information significant to older persons. Direct information included information for various older age groups (e.g., >65, >75, and >85), specific warning, specific adverse events, dose instruction, and pharmacokinetic changes in older persons. Indirect information included changes in dosage, drug-drug interactions, drug-disease interactions, information related to renal failure, and information related to liver failure for each PIL. The content analysis was classified as “yes or no,” when each of these items was present or absent, respectively. These criteria for the assessment related to content and layout were adapted, to the Portuguese language, from the study reported by Liu et al. 4.
The readability assessment was performed using the Flesch Reading Ease score (ranging from 0 to 100 with higher values corresponding to “easier to read the text”) and the Flesch-Kincaid Grade Level (which converts readability into years of schooling) 6. For PILs legibility, seven items were analysed: text font size <12; heading font size 14; maximum 5-6 bullet points in the included lists of information; unjustified right-hand margins; no italic fonts; no underlining; no capital letters 2.
For the present study, we considered “older people,” the individuals 65 years old or more, according to the definition of the Portuguese National Institute of Statistics 14. Data were analysed with IBM-SPSS software version 27.0 (SPSS Inc., Chicago, IL, USA). Quantitative data were analysed using descriptive statistics and are presented as mean, median, standard deviation, minimum, and maximum. The qualitative variables were described as counts (n) and percentages (%). Normal distribution for all the variables involved in statistical inference was assessed with Kolmogorov-Smirnov test. Considering the results of this test, parametric (Student’s t test, Pearson correlation coefficient) or nonparametric (χ2, Mann-Whitney’s test, Spearman’s correlation coefficient) procedures were used to analyse associations or group differences. Statistical significance for all procedures was set at p < 0.05.
Results
A total of 69 PILs corresponding to 41 (59.42%) antihypertensive agents, 9 (13.04%) oral antidiabetic agents, 11 (15.94%) antidyslipidaemic agents, and 8 (11.59%) anticoagulants and antiplatelet agents were included for the current analysis, considering the drugs identified in Table 1. The content of these PILs has been reviewed by the Portuguese marketing authorization holder, on average, 28.3 ± 21.5 months before the current analysis was carried out.
As presented in Table 2, the information about the use of medicines and its effects were included in most of the PILs (95.7%; n = 66) but no specific patients’ age range (e.g., >65 years, >75 years, >85 years) was referred in any PIL analysed. Specific precautions regarding the way the older population should use the medicines were available on less than half (46.4%; n = 32) of PILs included in this study. Additionally, the possible side effects that may occur in older persons were present in the form of advice but only in 7.2% (n = 5) of PILs and specific instructions about the recommended dose for people 65 years or more were available only in 27.5% (n = 19) of the PILs. It was also observed that a general recommendation for a follow-up during the initial phase of treatment was included in 13.0% (n = 9) of the analysed sample while information regarding the pharmacokinetic changes expected in the older population was included only in one PIL (1.4%).
Table 2 Number and percentage of PILs providing direct and indirect information specific for the elderly population

The content indirectly related to the use of medicines by the older population was also evaluated, and 5 parameters were considered (Table 2). Indications about possible dosage regimen modifications have been identified in less than half of the PILs (40.6%; n = 28). Potential drug-drug interactions and drug-disease interactions were described in 68.1% (n = 47) and 66.7% (n = 46) of the PILs, respectively, while specific details to be considered in patients with renal failure and/or hepatic failure were found in 49.3% (n = 34) of the PILs.
The number of brand medicines including specific direct information for older persons was higher compared to the generic medicines, although the difference is not statistically significant (p > 0.05). However, in the topics regarding “Information for various age groups” (e.g., >65, >75, and >85) and “Specific adverse events in the elderly” the number of brand medicines including specific direct information for older persons was lower compared to the generic medicines, despite no significant difference (p > 0.05).
Legibility assessment was carried out considering seven parameters as presented in Table 3. These parameters were evaluated considering only if each PIL complied with them or not. In some cases, the parameter “Maximum of 5-6 points in the included lists” was not applicable (29%; n = 20). It was observed that 43.5% (n = 30) of the analysed PILs were written using a text font smaller than 12, with no emphasis in the text content using underlined text (91.3%; n = 63) or capital words (40.6%; n = 28). Only two PILs met all seven legibility criteria considered, while only six complied with four parameters (in these cases, underlined or italic fonts and capital words were not used in the PILs).
Table 3 Number and percentage of PILs which comply or not with the parameters considered in the legibility assessment

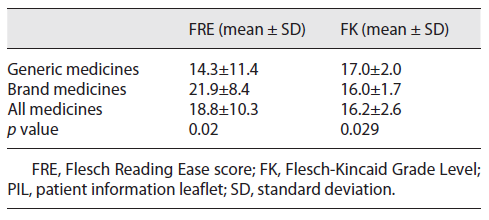
Readability was assessed using the Flesch Reading Ease score (FRE), and our results (18.8 ± 10.3) show that text associated with the sample of 69 PILs can be considered difficult to read. Furthermore, the results of the Flesch-Kincaid (FK) Grade Level suggest that readers should have completed 16.2 ± 2.6 years of schooling to be able to understand the information included in the PILs (Table 4).
A significant difference was observed in the FK grade level when considering PILs corresponding to medicines with different therapeutic indications. PILs related to oral antidiabetic drugs were easier to read, presenting a significantly lower FK (14.9 ± 1.0) and a higher FRE (28.8 ± 4.0), compared to PILs corresponding to antihypertensive drugs (17.0 ± 1.9 and 14.8 ± 10.8 for FK and FRE tests, respectively; p < 0.05). The mean value for readability was significantly higher (p < 0.05) in the FRE test for the brand medicines compared to the generic medicines (Table 4) which shows brand medicines PILs are easier to understand and that a lower schooling level is required for its comprehension (p < 0.05).
Discussion
The analysis of the PILs that was carried out in the present study showed that specific information for older persons is lacking in most of the analysed leaflets, particularly in direct information regarding adverse events, information for various groups, and pharmacokinetics. The information contained in the PILs is very extensive and diversified, and patients do not always look for integral content. The way they look for information may be different if it is the first time they use the medicine or if it is a usual use. According to a study held on a group of UK community pharmacies, the most searched information by the participants in the PILs is related to the possible side effects and to the details on how and when to take and therapeutic indication 15. However, according to the results of the current analysis, the information on this scope is presented in a general way, without proper information for groups aged ≥65 years old suggesting that even if these patients read the PILs they will not find the necessary information. A lack of information regarding side effects specific to older persons was also observed in a similar study held in the UK 4.
Another study, carried out in the North of Spain, determined that about a quarter of participants never read the medicines’ PILs. Notwithstanding that, the knowledge level about the information included in the PILs seems to be lower in older patients and in people presenting lower schooling levels, which means that older patients may be more vulnerable to the use of medicines 10.
Patients’ decisions, based upon PILs information, such as taking medicines at the right moment of the day, may be a consequence of reading its content (at the first use or later). An improvement in readability may improve leaflet reading and other corrective actions regarding the safe use of medicines could arise with positive outcomes for patient’s health 15.
The information most searched in the PILs is mainly that related to side effects, details on how and when to take, and therapeutic indications 4,16. However, specific information for older persons and related to these points is still scarce, as verified in the current study (Table 2).
Ageing is responsible for several changes in the human body which may have an impact on drugs pharmacokinetics properties, including the bioavailability of the drug, its therapeutic action, and increasing the odds of adverse events (including those caused by drug-drug interactions) 16,17. In the current study, information regarding the changes in pharmacokinetics for older patients was only found in a very small number of PILs (1.4%; n = 1). In addition, the dose instructions specific for older persons were only found in about a quarter of the PILs analysed (27.5%, n = 19), which means that patients need to remember the doctor’s prescription and can not rely on PILs if they forget the information.
In the Portuguese population, several problems were highlighted in relation to general literacy including limited reading habits and writing practices. The results of a national health literacy survey show that participants referred to difficulties in understanding the information contained in the medicines information leaflets, among other documents, with consequent limitations to being able to decide about treatment options. Also, the results of this survey indicate that advanced age and lower schooling levels were associated with the poorest health literacy levels 18.
According to the available data, in 2020, about a third of the Portuguese population (29.8%) still has less than 9 years of schooling (although the current minimum education level established in the Portuguese law is 12 years since 2009) 19,20. Approximately 10% of the population just completed the first 4 years of schooling, and in this group is included a significant proportion of the people with 65 years old or more 19. Considering that the results of the current PILs’ analysis indicate 16 years of schooling as a requirement to understand the information contained in the PILs, there is an important difference between the educational requirements needed to understand the PILs’ content and the educational level of the older Portuguese population. These difficulties were also clear in a study carried out in a medical imaging service of a central hospital in Portugal which showed that most of their users do not have the desirable literacy to read the hospital flyers, expressing the need to rely on other people to help them to understand the information 21.
The fact that PILs are mostly written in plain text, without using bullets, bold, or underlined text to highlight specific items of its content (Table 3), can negatively affect the readability and interpretation of the contained information. In a previous analysis carried out in Portugal regarding the evaluation of PILs of nongeneric medicines, a “long length of the text” has already been pointed out as a negative factor for understanding the information 22.
It has been observed that older patients, particularly those with lower educational levels, have preferences for less information, for coloured text, and for text using symbols 5. The use of colours in the PILs seems to improve the navigation across different sections of the document, although it seems that it does not significantly improve the time needed to find the information, compared to the black-and-white version 23.
It is common knowledge that reading the names of medicines is difficult. Tong et al. (2020) developed a methodology to assess patient-centred prescription label formats, having clearly identified that some formatting characteristics affect the ability of individuals to identify relevant information in the medicine, such as the distinction between the name of the active substance and the trade name of the medicine 24. This issue is mitigated in generic medicines as they are designated by the active substance. In Portugal, there has been an increase in the consumption of generic medicines during the last decade, which is economically very positive to reduce health care costs 25. However, according to the results of the current study, the readability of PILs of generic medicines is lower than that of brand medicines, and this fact may not be a favourable contribution to understanding the information about the medicines consumed.
Although European legislation still requires the inclusion of a printed PIL in the packages of all medicines, currently it is easy to access these documents in electronic format. However, according to a group of Swedish pharmacy customers, the preference is still for the traditional paper format, despite identifying the electronic format as positive 26. This reinforces the need to produce adequate and readable PILs that easily provide the required information to patients of all ages and educational levels. In addition, consulting health professionals to obtain information about medicines is still a very important option, particularly for older persons 27, but it should be considered that younger generations, with higher literacy levels and more used to use the Internet, will get older and will also need to have access, both in paper or electronic format, to the information contained in the PILs.
The use of tailored written information has received a positive evaluation from the user’s perspective, as observed in a pilot Portuguese study that used software to produce individual patient leaflets with information related to some prevalent diseases 28. In the future, the availability of tailored written information about medicines, considering different sources, may be an alternative approach contributing to improving health outcomes. In 2020, the European Medicines Agency already launched the key principles for electronic product information to be prepared for medicines in the European Union 29. The information available in the PIL could be adaptative, depending on patients’ characteristics. Further studies in which the information is adapted according to the individual’s characteristics (using algorithms that could become available to the pharmacists when dispensing medicines) are needed. This could also be useful to adapt the information and respective tools to be used by the health professionals to contribute to improving the health literacy of the general population and the health outcomes obtained by each patient.
Statement of Ethics
There was no approval by the Ethics Committee as this analysis did not include personal data. An ethics statement was not required for this study type as it is based exclusively on published literature.
Conflict of Interest Statement
All authors declare to have no conflicts of interest associated with this publication.