Introduction
Malignant mesothelioma is an uncommon, difficult to diagnose, and a poorly prognostic type of cancer that occurs in the mesothelium (pleura, peritoneum, pericardium, tunica vaginalis, and testis) 1-5. It occurs most often in males and those aged 65 and older 1,3, has a long latency period associated with 30-50 years 1,6, and the survival rate is estimated to average 9-12 months after diagnosis 2,3.
In 1960, Wagner presented a study that demonstrated the causal relationship between pleural mesothelioma (PM) and exposure to asbestos 7 leading to today´s recognition of asbestos as a human carcinogen in all its varieties 8, and PM being monofactorial to asbestos exposure 1,9-11 and the most common form of all mesotheliomas 9,12, as a determinant factor for its study.
Incidence and mortality rates of PM reflect the high production and consumption of asbestos in industrialized countries in the past 13,14. In this sense, and because there is still much to know about these data, dedicated registers are beginning to appear around the world 15,16. The use of standardized questionnaires is one of the tools used by some of these registries, namely in France, Italy, and Australia 15 that can help characterize asbestos exposure, study other potential etiologic factors, improve diagnoses of PM, and even assess the recognition of PM as an occupational disease.
In Portugal, despite the use/marketing of asbestos and/or products containing it being banned in 2005 17, there is still no specific registry for PM and consequently, no standardized questionnaire validated for the Portuguese context. Thus, the main objective of this study was to describe the translation, cultural adaptation, and content validation process of the French National Surveillance Programme for Pleural Mesothelioma (FNSPPM) questionnaire, a standard questionnaire, used in the French program for the surveillance of PM, which has made it possible to establish the epidemiological evolution of PM in terms of incidence, survival, exposure, and medical-social recognition since 1998 11, for the Portuguese context.
Materials and Methods
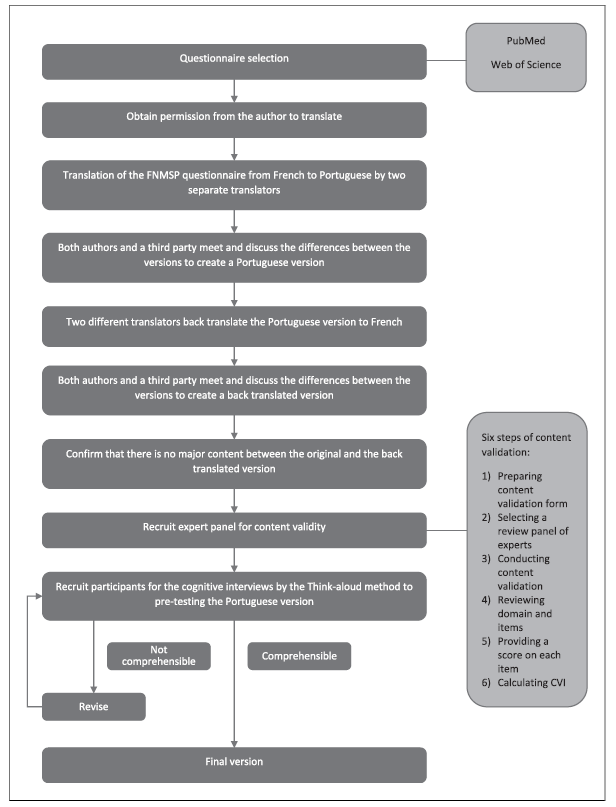
This study was developed in two phases: phase I - questionnaire selection by searching databases and phase II - translation, cultural adaptation, and content validation of the FNSPPM questionnaire for the Portuguese context (Fig. 1). The STROBE guidelines for observational studies 18 were followed.
Phase I - Questionnaire Selection
Using the descriptors questionnaire, asbestos, and PM, a search was conducted in the PubMed (using the terms MESH) and Web of Science databases for the period January 1, 1960, to December 31, 2022. We extended our research to 1960 because it was the year in which the link between PM and asbestos exposure was demonstrated for the first time 7.
The research was conducted from 1 to January 5, 2023, and included all studies that mentioned questionnaires related to asbestos exposure (occupational and nonoccupational contexts) and PM in humans. We restricted our search to studies written in English and French. For each questionnaire found, a search was performed in the respective country registry to get more information. All questionnaires written in English and French designed to be applied to humans with PM and both occupational and nonoccupational asbestos exposure were included.
Data were always reviewed by two reviewers (C.S. and E.S.L.), and a third reviewer (A.S.U.) read studies to resolve disagreements. Both reviewers independently extracted study data into a prepared spreadsheet (Excel®, Microsoft Corporation, Redmond, WA).
Phase II - Translation, Cultural Adaptation, and Content Validation
Permission to translate, culturally adjust, and validate the FNSPPM questionnaire in the Portuguese context, was obtained from the original authors (team leader Patrick Brochard, Anabelle Gilg Soit Ilg, and Marcel Goldberg) via e-mail.
The Instrument: FNSPPM Questionnaire
After the research was conducted and based on the inclusion criteria, the FNSPPM was the only questionnaire that met the inclusion criteria. It is a questionnaire, composed of 69 questions, divided into 2 domains: the first, composed of 13 questions, refers to the characterization of the individual (identification, first job, place of first residence, educational establishment, list of jobs held by spouse, and parents) and interviewer and interview details; the second, composed of 56 questions (31 main questions and 25 sub-questions), to occupational and nonoccupational characterization, habits, and health (specific activities, specific factors, environment, family environment, family background, tobacco, and personal background). In questions 1 to 18, if the answer is “yes,” the respondent is also asked to fill in a table, which allows a more detailed characterization of the asbestos exposure. The FNSPPM questionnaire, created in 1998, is applied by interview and subsequently evaluated by experts by an industrial hygienist and an occupational physician which assess the type of exposure 9.
Translation Process
FNSPPM was translated by World Health Organization (WHO) best practice guidelines 19. Initially, it was forward translated into Portuguese, by two independent individuals fluent in both Portuguese and French. The backward translation was done also by two independent individuals fluent in both languages, who had never had contact with the original one. Any variances in the translation and backward translation were settled by consensus and with the help of a third party, who was also fluent in both languages. The consensus back-translation was then compared to the original FNSPPM questionnaire to ensure that no conceptual losses occurred during the process.
Expert Panels
To support the validity of an assessment tool such as questionnaires, it is important to establish the content validity that can be represented by the content validity index (CVI). This should be a systematic and rigorous process, which should respect six steps: preparing content validation form; selecting a review panel of experts; conducting content validation; reviewing domain and items; providing a score on each item; and calculating CVI 20. The number of experts for content validation should be at least 6 (CVI values of at least 0.83) and not more than 10 (CVI values of at least 0.78) 20.
In this sense, and to initiate this process, a CVI questionnaire was created on Google Forms, using a Likert scale (1-4), which allows experts to critically evaluate the relevance of each question 20 and, in the same questionnaire, through free questions, give suggestions regarding the content and semantics. This CVI questionnaire and the first version of the FNSPPM questionnaire for the Portuguese context were sent via e-mail to nine experts (n = 9) in Occupational Health, Public Health, Pulmonology, Epidemiology, Oncology, and Anatomopathology. They were recruited (convenience sample) in July 2023 and had, as inclusion criteria, to be health professionals (nurses and physicians), with a minimum of 3 years of practice in their areas of specialty, exercising functions in care provision (hospital and primary health care) and/or in higher education.
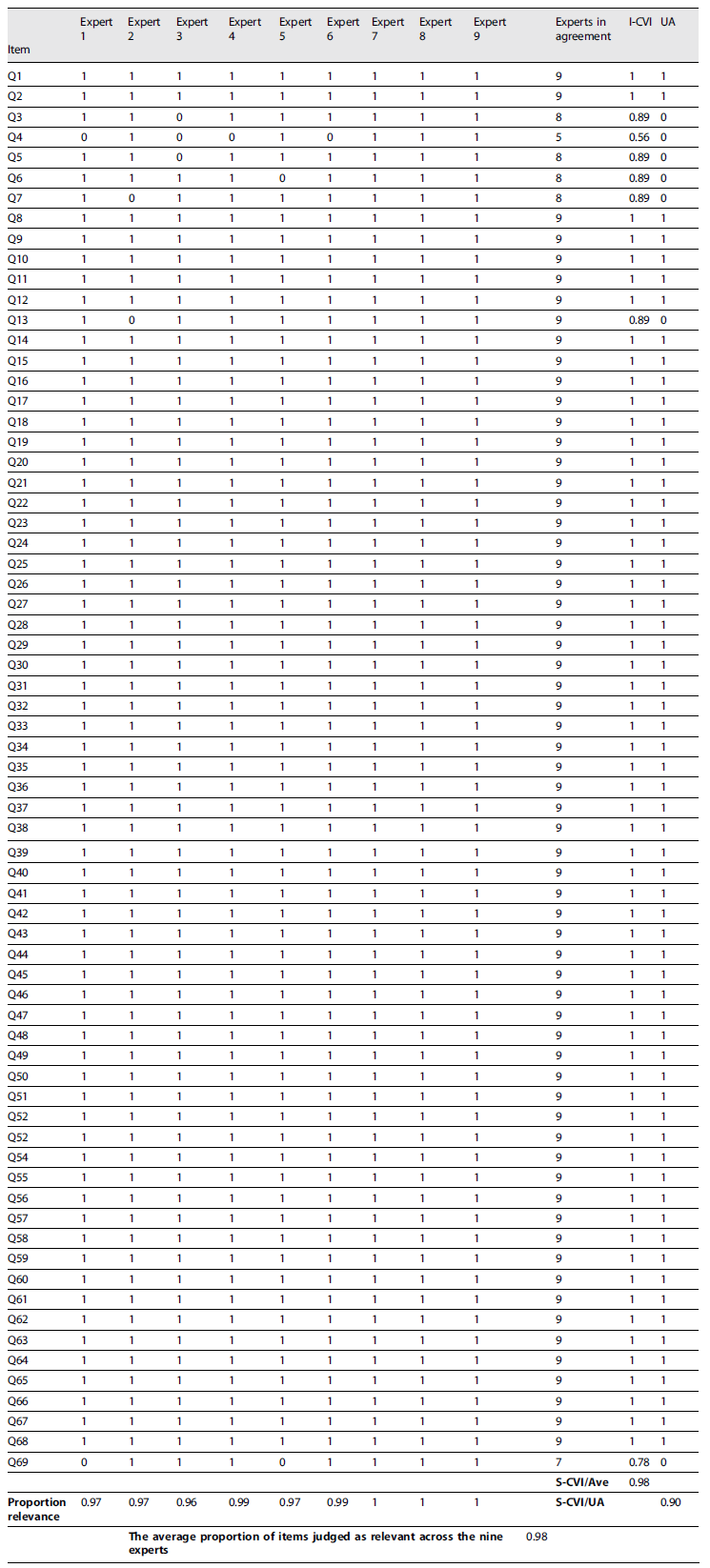
After the CVI questionnaire was completed and submitted, the questions assessed with relevance 3 and 4, were recoded as 1 and those assessed with relevance 1 and 2, were recoded as 0. To ensure that the questionnaire has achieved a satisfactory level of content validity, item-level content validity index (I-CVI), scale-level content validity index based on the average method (S-CVI/Ave), and scale-level content validity index based on the universal agreement method (S-CVI/UA) were calculated. To calculate the universal agreement (UA) score is given as 1 when the item achieved 100% experts in agreement, and a 0 if this is not the case. Questions with CVI lower than acceptable may be adjusted or excluded. Data were statistically treated using Excel® (Microsoft Corporation, Redmond, WA).
Pretesting
Pretesting is fundamental in the validation of a questionnaire before its application, insofar as this minimizes misunderstanding and subsequent measurement error and consequently allows participants from the target population to also contribute their knowledge 21. Cognitive interviews using the Think-aloud method, is one of the recommended techniques, applied to around 5-15 individuals, in 2 to 3 rounds or until saturation 22. The interviews were applied to a total of 10 participants (n = 10), divided into two rounds. The participants were recruited (convenience sample) in August 2023, individuals over the age of 18, of Portuguese nationality, and residing in Portugal, with at least one known exposure to asbestos in occupational and/or nonoccupational context.
The interviews were separately conducted by two authors (C.S. and E.S.L.) during August 2023 through digital interviews. Before the interviews, participants received information about the study and provided informed consent. Each item of the FNSPPM questionnaire for the Portuguese context was displayed to the participants to allow them to spontaneously evaluate if the questions were a good fit in the Portuguese context.
Cultural Adaptation
The cultural adaptation was made during the previous steps, by removing information considered sensitive for data protection (address) and by adjusting certain concepts to respect the gender of each individual (masculino/male; feminino/female; não binário/nenhum/non-binary/none; prefiro não responder/prefer not to answer; outra/other) and to the Portuguese context in particular schooling (nenhum/none; básico 1° ciclo/basic 1st cycle; básico 2° ciclo/basic 2nd cycle; básico 3° ciclo/basic 3st cycle; secundário/pós-secundário/secondary/post-secondary; superior/higher education) 23 (Table 1).
The Final Version
After analyzing the original questionnaire, and the results obtained through the expert panel and cognitive interviews, the authors reached a consensus. Some items were reworded to make them more understandable or excluded, but every precaution was taken to keep them as close as possible to the original questionnaire.
Results
Questionnaire Selection
The database search yielded 215 hits (PubMed n = 159 and Web of Science = 56). Assessment of selected studies’ reference lists identified no additional eligible studies. A total of 185 unique papers were identified and screened. 6 questionnaires from Australia, France, Italy, Spain, South Korea, and Turkey were identified in 38 of those studies.
Of the 6 countries identified, only 4 questionnaires were in the languages defined as inclusion in this study, namely Australia, Spain, France, and Turkey. After the analysis of the articles and access to the registries of the respective countries, the Australian is a web application (OccIDEAS) aimed at mesothelioma in general 24, the Spanish, despite being in English, aimed at establishing the probability of exposure to asbestos and to asbestos-related diseases 25, the Turkish, despite being in English, only addressed exposure to asbestos in a nonoccupational context 26, and only the FNSPPM questionnaire meet the inclusion criteria since it is in French and is aimed at humans with PM with asbestos exposure, regardless of occupational and nonoccupational context.
Translation Process
In step 1, the French version was translated into Portuguese. For cultural adaptation, questions about the background characteristics of the participants regarding schooling were adapted to the Portuguese context. Questions that called into question the anonymity of individuals or institutions, such as addresses, were excluded as they did not respect data protection and sensitive data such as gender have been redacted 27. In step 2, the Portuguese version was translated back into French. No changes are required. From this process, we obtained the first version of the questionnaire in the Portuguese context.
Expert Panels
The nine experts gave their comments on the CVI questionnaire and rated each item’s relevance. 68 out of 69 questions, had an I-CVI >0.78, which led to an S-CVI/Ave of 0.98 and an S-CVI/UA of 0.90, which was interpreted as relevant ((Table 2). Only item Q4 - “local de nascimento: freguesia/place of birth: parish,” had an I-CVI of 0.56, and after analysis by the authors, it was excluded because it could jeopardize the anonymization of the participants 27. Question 10 was rewritten according to the expert’s suggestions, with professional activity and company activity being coded according to the Portuguese Classification of Economic Activities 28 and professional exercise/professional category being coded according to the Portuguese Classification of Occupations 29. From this process, we obtained the second version of the questionnaire for the Portuguese context.
Pretesting
The ten participants of the Think-aloud process reported understanding the questionnaire and its instructions. No adjustments to the translation and context were necessary. From this process, we obtained the final version of the FNSPPM questionnaire for the Portuguese context (online suppl. file 1; for all online suppl. material, see https://doi.org/10.1159/000538097).
Discussion
Summary of Evidence
In this study, we developed the first version of the Portuguese context of an existing questionnaire for the study of exposure to asbestos and PM. The importance of having such a questionnaire is because they are fundamental tools for establishing exposure to asbestos 30.
In the search conducted in the databases, references to 6 questionnaires were identified, from Australia, France, Italy, Spain, South Korea, and Turkey. Of the 6 countries identified, Australia, France, South Korea, and Italy have a National Mesothelioma Registry 15, Spain has a Mesothelioma Mortality Registry 31 and Turkey created a mesothelioma database between 2008 and 2012 15. Of these 6 questionnaires, only 4 were in the language defined as inclusion criteria but only the French questionnaire met all the inclusion criteria.
Created in 1998, the FNSPPM covers currently around 30% of the French population and uses the FNSPPM questionnaire, also developed in 1998, as a tool 11. In this sense, during the translation process, close attention was paid to ensuring that the translated version matched the original version to ensure validity 19. A content validation procedure was performed by a panel of nine experts, obtaining I-CVI values higher than 0.78 in 68 of the 69 questions, an S-CVI/Ave of 0.98 and an S-CVI/UA of 0.90, which demonstrates that the items are relevant and that there is agreement among the experts on the relevance of the questionnaire for application in the Portuguese context. The participants of the Think-aloud process reported understanding the questionnaire and its instructions, with no adjustment necessary. No study was found to support these statements. However, given that the questionnaire has been used for over 20 years by an organized and functional program 11, the content validation levels are satisfactory, with I-CVI, S-CVI/Ave, and S-CVI/UA values higher than 0.78 20, and the cognitive interviews by the Think-aloud method are highly recommended 22, we may consider the questionnaire to be relevant to the Portuguese Context.
Alongside the importance of using standardized questionnaires, the creation of dedicated registries has also been highlighted since to have a better knowledge of the reality of asbestos consumption/production and the incidence and mortality rates due to PM is fundamental, as well as the fact that studies show that incidence is not decreasing as expected, new cases still occur and mortality has not substantially changed over time 15. To date, despite all the efforts to ban the use of asbestos, only 67 countries have joined the practice 32 and many countries still consume and produce asbestos, which means this will continue to be a major health problem for many decades.
Implications for Future Research
The existence of a questionnaire will allow a better understanding of the exposure to asbestos in Portugal and support for the prevention, early detection, and treatment of PM.
Limitations
The main limitations of this study were that the search conducted to choose the questionnaire excluded publications that were not in English or French, and during the selection of studies, studies of interest from other languages were identified, as well as the fact that it was not possible to apply the questionnaire to a more substantial sample of patients with PM.
Conclusion
The results of this study indicate that the Portuguese version of the FNSPPM questionnaire has achieved a satisfactory level of content validity and therefore could be used in the study of individuals with PM and exposure to asbestos in a Portuguese context. Furthermore, even though the questionnaire seemed relevant, more work must be performed to apply the questionnaire to a significant sample of the Portuguese population and for the creation of a specific registry to study this serious Public Health problem in Portugal and the world.
Acknowledgments
We would like to thank all the experts who participated in the translation, cultural adaptation, and content validation of the questionnaire for their valuable contribution.
Statement of Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki 33 and approved by the Ethics Committee of the National School of Public Health (n.o 22/2023). Written informed consent was obtained from all participants. The guarantor of this article (C.S.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. We plan to disseminate the findings and conclusions from this study through scientific conferences.
Conflicts of Interest Statement
The authors have no conflicts of interest to declare.
The authors declare that no experiments were performed on humans or animals for this investigation. The authors declare that no patient data appears in this article.
Author Contributions
Conceptualization: C.S., E.S.‐L., P.A., and A.S.‐U.; data curation and formal analysis: C.S. and E.S.‐L.; methodology and validation: C.S., M.A.D., S.F., E.S.‐L., and A.S.‐U.; resources and writing - original draft: C.S; supervision and review and editing: M.A.D., S.F., E.S.‐L., P.A., and A.S.‐U. All authors revised the manuscript and approved the final version. C.S. is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and no others meeting the criteria have been omitted.