Introduction
Alopecia Areata (AA) is an autoimmune disorder characterized by nonscarring hair loss with preservation of the hair follicle, affecting up to 2% of the general population and imposing a significant psychological impact on affected patients1.
AA can present in multiple patterns, and its severity may range from episodes of well-defined patchy hair loss on the scalp to the more serious and complete scalp (alopecia totalis) and total body hair loss (alopecia universalis)2.
The theory of loss of the immune privilege of the hair follicle is thought to play a crucial role in AA3. The presumed target antigen is yet to be defined. The succeeding loss of hair follicle immune privilege results in the recognition of hair follicle autoantigens by autoreactive CD8+ T cells4. Patients with AA have a constellation of potentially associated autoimmune conditions and an increased risk of Atopic Dermatitis (AD)3.
Common treatment options for extensive AA, where intralesional and topical treatments are often of restricted applicability, include systemic immunosuppressants like methotrexate and cyclosporin, which generally provide temporary responses and are unsuitable for long time use5. Thus, there is a great demand for new, specific treatments for long-term disease control in moderate-to-severe AA.
Indeed, recent evidence suggests that Dupilumab, a monoclonal antibody directed against the IL-4 receptor α subunit blocking both IL-4 and IL-13 signaling and currently approved for the treatment of atopic dermatitis, could be of some benefit in AA6.
Baricitinib, a Janus kinase inhibitor, has been approved for severe AA and other drugs from this class are presently undergoing clinical trials with positive preliminary results7.
Cases have been reported of the improvement of AA in patients with concomitant AD receiving treatment with dupilumab8.
The effect of dupilumab in AA has been postulated to be the inhibition of Th2 pathway activation found in AA scalp lesions9. Nevertheless, dupilumab has also been associated with AA both in patients with preexisting AA and those without a prior diagnosis of AA10.
Case synopsis
A 29-year-old man was evaluated due to uncontrolled AD. He had a history of recurrent flares of AD since childhood with worsening in the last 2 years. He also had a 6-month history of round patchy hair loss.
He had AD from the age of 3, with severe itching, sleep deprivation, and a great impact on his quality of life and was on an almost persistent use of topical corticosteroids and calcineurin inhibitors and occasional courses of systemic therapies with prednisolone as well as cyclosporin with limited and transient benefit. He reported asthma in childhood with spontaneous improvement and no current need for therapy.
Of relevance, he also had an episode of eczema herpeticum and herpetic keratoconjunctivitis treated with intravenous acyclovir a couple of weeks prior to the observation.
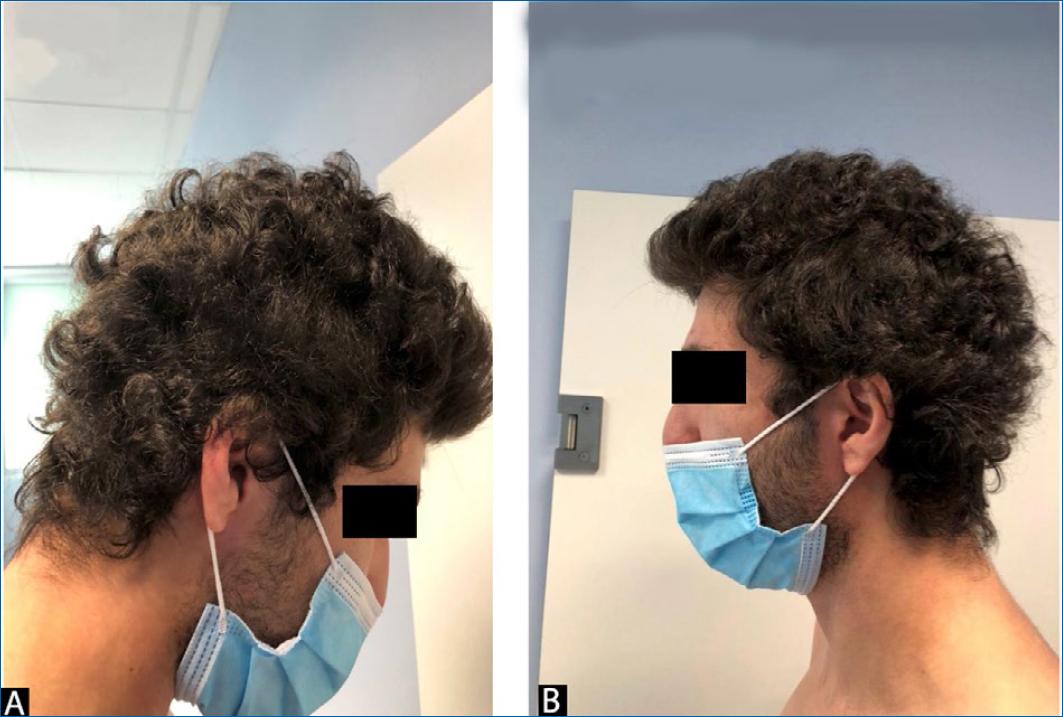
On physical examination, multiple erythematous papules and plaques, some of which were severely excoriated by scratching, were found on the entire skin including the face, cervical region, flexures of the forearm, and knees. EASI score was 14,6. He also had several patches of alopecia on occipital, temporal, and parietal regions, with scaling and erythema (Fig. 1). The eyebrows, eyelashes, and body hair was spared.
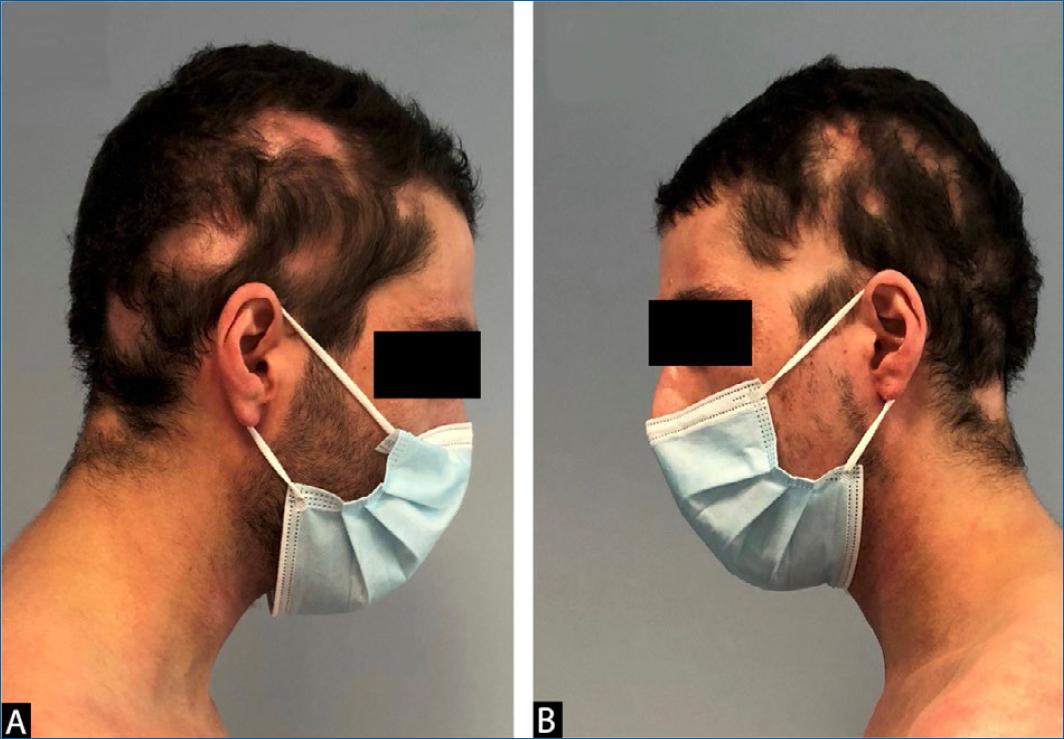
Figure 1 A and B: patches of alopecia on occipital, temporal, and parietal regions, with scaling and erythema.
Laboratory tests were normal (hemogram with differential blood count, kidney, liver and thyroid function, and electrolytes) except for increased serum IgE levels (3055.3 IU/mL). Trichoscopy showed yellow and black dots and a perifollicular scale. Two 4 mm punch biopsies were performed on the scalp, showing dermis with adnexal rarefaction, presence of a sebaceous component, and fibrosis with a mild lymphocytic infiltrate and “stela” like structures, favoring the diagnosis of alopecia areata.
He was started on dupilumab with an initial dose of 600 mg followed by 300 mg every 2 weeks.
The patient was observed to evaluate the response 16 weeks after the first dose of dupilumab, with a dramatic improvement regarding atopic dermatitis with an EASI drop close to 0 and a resolution of the itching, a better sleep pattern and no need for any topical therapy except emollients. He also had full hair regrowth in the areas previously affected by AA (Fig. 2).
Except for slight conjunctivitis, which was successfully treated with artificial tears, the patient had no side effects and so far has tolerated the medication extremely well.
Discussion
Patients with AA show elevated levels of type 2 cytokines suggesting that Th2 axis suppression may be a therapeutic target for AA9. As dupilumab-induced IL-4 inhibition results in a decrease in inflammatory mediators, it likely leads to concomitant improvement of both AA and AD11.
According to previous reports, hair regrowth with dupilumab usually begins between 3 and 6 months of therapy, although it may begin earlier12.
However, the relationship between dupilumab and alopecia areata remains controversial as some patients experience improvement after starting treatment while others develop dupilumab-induced alopecia13 with some studies suggesting that Th2 inhibition may amplify alternate pathways such as Th1 and Th17 resulting in hair loss14. Moreover, alopecia associated with dupilumab tends to follow an androgenetic pattern and usually has an eczematous pattern upon histopathological examination15. It is unclear whether dupilumab induces true AA or a form of drug-induced alopecia.
Janus Kinase Inhibitors have shown efficacy for moderate-to-severe AA16. In this case, baricitinib could have been an option due to the coexistence of AA and AD. However, due to the recent eczema herpeticum, we opted for dupilumab due to its safety and were extremely pleased by the effect on hair regrowth.
However, we should highlight that the temporal relationship between dupilumab use and AA hair regrowth does not ensure causality, as there may be spontaneous AA improvement. Therefore, we cannot attribute this fast improvement to dupilumab with certainty.
A study using the VigiBase, a real-world pharmacovigilance database concluded that hair disorders corresponded to 2.2% of total dupilumab adverse events. This study concluded that dupilumab was associated with both hair growth and loss and overall, the paradoxical results of dupilumab in hair disorders might be caused by complex disease factors, such as IgE and IL-4 levels and the complexity of AA pathogenesis14.
A clinical trial has recently demonstrated the pathogenic role of the Th2 axis in AA and the efficacy of dupilumab in moderate-to-severe AA17. Furthermore, this trial showed that patients with IgE ≥ 200 IU/mL had an odds ratio of 8.1 to respond to dupilumab treatment compared to those with low baseline IgE, suggesting that IgE may be a tool for patient selection for dupilumab treatment, a new concept in AA management. A possible explanation for these findings may be that increased levels of serum IgE are indicative of Th2 -associated inflammation in the hair follicle, which may respond better to Th2-targeting approaches17.