Introduction
Central centrifugal cicatricial alopecia (CCCA) was originally described in 1968 as “hot comb alopecia,” due its association with common hair grooming practices in the African American community1,2. Since then, advancements have significantly enhanced our understanding of CCCA’s epidemiology, pathogenesis, and management strategies, though many aspects remain enigmatic.
CCCA is considered primary cicatricial alopecia (PCA), wherein hair follicle destruction results in irreversible fibrosis and scalp scarring1. According to the North American Hair Research Society’s classification system, PCAs are classified by the predominant inflammatory cell infiltrate – lymphocytic, neutrophilic, mixed, or nonspecific2. Conventionally, CCCA and lichen planopilaris (LPP) are considered as distinct lymphocytic PCAs. A classical review recognizes three clinical patterns within LPP – classic LPP, Graham-Little-Piccardi Syndrome, and frontal fibrosing alopecia (FFA)2. However, some authors hypothesize that CCCA may be a diffuse form of LPP or part of the same continuous spectrum within lymphocytic PCA, occurring in a specific racial group, as they are nearly identical both clinically and histologically3. In this article, we explore this theory through a detailed review of recent research, highlighting the similarities and differences between these two entities.
Epidemiology
CCCA is the most common type of PCA in women of African descent, although the real prevalence remains unknown and the condition is underdiagnosed and underreported4. It is rare in African males, with an estimated female: male ratio of 3:15,6. Since symptoms of CCCA are frequently absent, diagnosis is often delayed, and patients typically present during the third and fourth decades of life, after irreversible scarring has occurred1. Although uncommon in children, biopsy-proven cases of pediatric CCCA have been reported in the literature5.
Patients with CCCA are at higher risk of developing other comorbidities including fibroproliferative disorders, breast cancer, and metabolic syndrome, particularly type II diabetes mellitus (DM2)1,6.
Compared to LPP, both conditions share a similar age and gender prevalence – primarily young adult females2. The main difference is that LPP is more prevalent in Caucasians, whereas CCCA occurs only in African descent2.
Pathogenesis
The etiology and pathophysiology of PCA are poorly understood.
In CCCA, the pathogenesis is believed to involve a complex mixture of a multitude of factors including but not limited to genetic predisposition, variants in gene expression, disruption of the balance between proinflammatory and anti-inflammatory factors, and ethnic hair care practices5.
Considering the genetic basis, CCCA has been demonstrated, in some families, to be inherited in an autosomal dominant pattern with partial/variable penetrance4. New research suggests that pathogenic variations in the gene encoding peptidyl arginine deiminase type III (PADI3) have been implicated in the pathogenesis of CCCA. PADI3 is an enzyme responsible for the posttranslational deimination of proteins crucial in hair shaft formation1,5. Missense and splice PADI3 gene mutations may result in decreased enzymatic activity, misfolded proteins, and abnormal protein localization, ultimately leading to decreased hair follicle development and hair shaft malformations1,5.
In terms of the imbalance between pro-inflammatory and anti-inflammatory factors, matrix metalloproteinase 9 (MMP9), a key biomarker associated with fibrosis pathways, has been demonstrated to be upregulated in severe cases of CCCA. Clinically, this dysregulation manifests as low-grade inflammation observed in dermoscopy and histopathology, which progressively leads to fibrosis6.
The specific role of hair grooming practices remains unclear with some studies finding no association, and others identifying certain grooming practices, such as traction and chemical styling, that may aggravate or trigger the condition1,7.
The pathogenesis of LPP is thought to involve T-cell-mediated autoimmune targeting of unknown antigens in the follicular bulge, with subsequent destruction of the hair follicle8. It is proposed that this reaction is potentially initiated by the action of an endogenous or exogenous agent, which binds to keratinocytes as well as follicular epithelium. Thereafter, keratinocyte and hair follicle may act as signal transducers, capable of converting these stimuli into producing cytokines and chemotatic factors for initiation of inflammation and production of interferon-gamma, tumor necrosis factor-alpha, and enhancing antigen-presenting cell/T-cell interactions9. Exogenous factors proposed as potential risk factors for the development of LPP include contact sensitizers (gold, mercury, cobalt), viruses and bacteria (hepatitis C virus, Helicobacter pylori, herpes simplex virus type 2, human immunodeficiency virus, human papillomavirus, and syphilis), trauma, and medications (angiotensin-converting enzyme inhibitors, β-blockers, thiazides, quinidine, and antimalarials)8. Unlike CCCA, there is no strong evidence of association with genetic factors for LPP8.
Clinical presentation
Classic active LPP presents as single or multiple coalescing patches of alopecia, typically at the vertex and parietal scalp, with perifollicular erythema and scaling at the active edge, associated with trichodynia and itching (Fig. 1A)8.
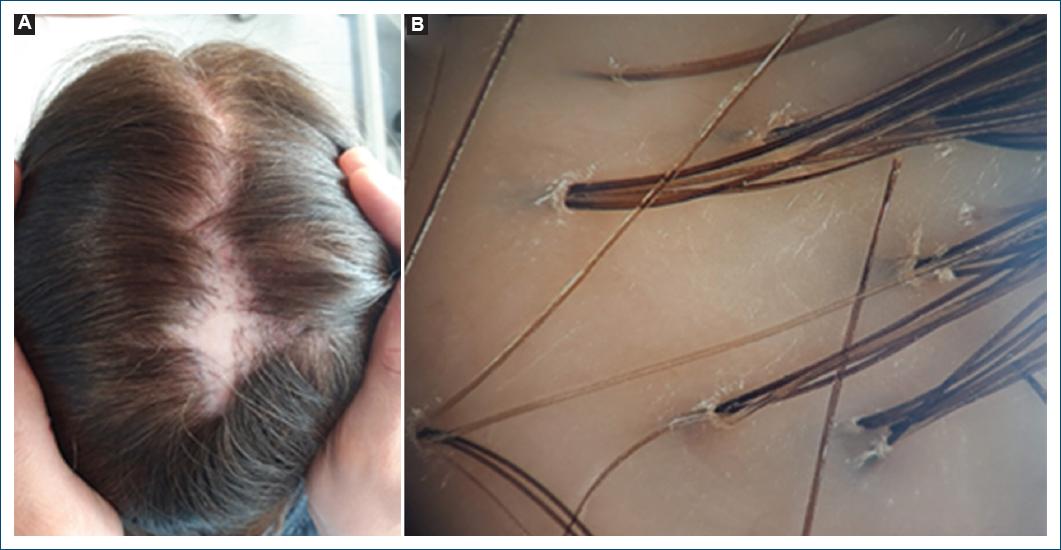
Figure 1 Classic lichen planopilaris. A: clinical picture showing extensive multifocal, interconnected alopecic patches with perifollicular erythema, in the crown. B: trichoscopy revealing peripilar silver scale with tubular hair casts, and polytrichia.
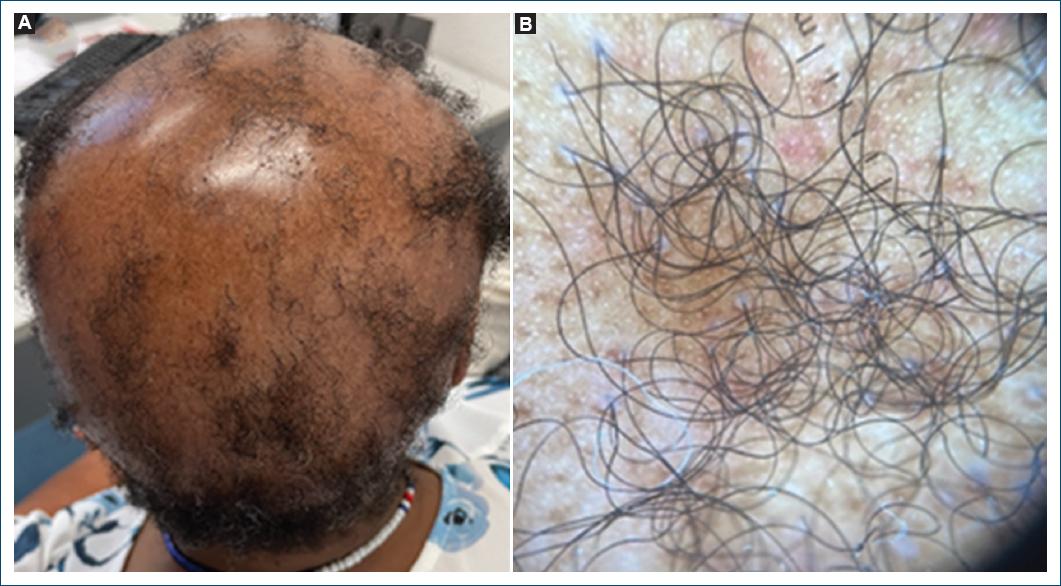
On the other hand, CCCA begins as an area of decreased hair density or hair breakage notably centered at the vertex of the scalp and expands peripherally (Fig. 2A)2,5. Similar to LPP, in early stages, CCCA lesions may also be pruritic or tender, although symptoms are much rarer and minimal inflammation is typically appreciated clinically1,2. A patchy form of CCCA has recently been described, highlighting the clinical variability that can pose diagnostic difficulty5. Advanced cases reveal impressive hair loss evidenced by a smooth, shiny scalp with loss of follicular ostia5.
Trichoscopy
The loss of follicular openings occurs in both CCCA and LPP as they are types of PCA.
A peripilar whitish-gray halo corresponding to concentric peripilar fibrosis is considered the most specific trichoscopic finding and highly sensitive for early and late-stage CCCA1. Erythema and peripilar scaling and broken hairs are shared with LPP, although they are milder (Figs. 1B and 2B)3. In LPP, prominent tubular hair casts can be observed3.
Additional trichoscopic features of CCCA include a disrupted honeycomb pigmented network and irregularly distributed pinpoint white dots3. It should be noted that the honeycomb pigment network, corresponding to hypomelanotic areas overlying dermal papillae separated by hyperpigmented lines of epidermal ridge, and the pinpoint white dots, corresponding to eccrine duct openings, are normal trichoscopic findings in dark scalp skin in which CCCA exclusively occurs3. Thus, the disruption and irregular distribution of these findings in CCCA normally pertain to dark-skinned patients3.
Furthermore, some cases of CCCA present hair follicle miniaturization and hair shaft variability with peripilar white halos visible by trichoscopy, as seen in fibrosing alopecia in a pattern distribution (FAPD), a mild variant of LPP incorporating androgenetic alopecia (AGA), suggesting that these cases of CCCA may be FAPD in patients of African background (Fig. 3)3.
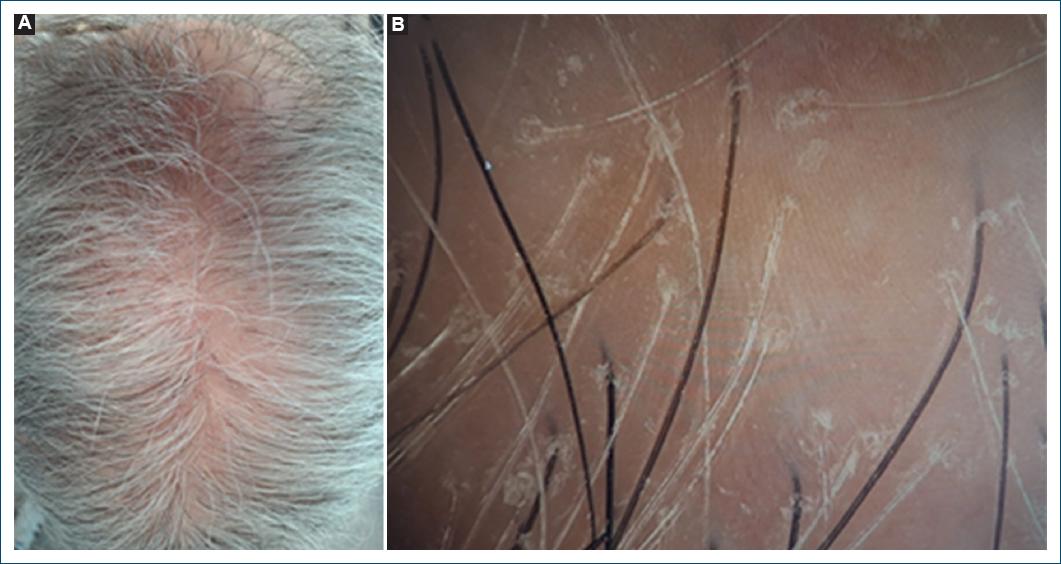
Figure 3 Fibrosing alopecia in a pattern distribution. A: clinical picture of hair loss on the crown resembling androgenetic alopecia. B: trichoscopic findings: anysotrichia, coexistence of vellus and terminal hair, mild peripilar scaling, and micro-islands with no follicular orifices (scarring alopecia).
Histology
Lymphocyte-predominant PCA, including LPP and CCCA, share several histopathologic features: blue/gray-staining perifollicular fibrosis at the infundibulo-isthmic portion of the hair follicle, a perifollicular lymphocytic infiltrate associated with the fibrosis, compound follicles, eccentric epithelial atrophy, eccrine duct dilation, premature desquamation of the inner root sheath (PDIRS) of inflamed follicles, and loss of sebaceous glands2.
In the active stages of CCCA, there is follicular lichenoid inflammation, bit in the later stages, follicular fibrosis prevails6.
The histopathologic feature considered most consistent and characteristic of CCCA is PDIRS, opening a potential route for external irritants to penetrate deep into the follicle, namely during hair grooming practices7.
A prospective, blinded study published in 2005 compared various types of PCA and illustrated the diagnostic limitations among non-lupus lymphocyte-predominant PCA (classic LPP, FFA, pseudopelade of Brocq, and CCCA). This study demonstrated that while histopathologic features help distinguish lymphocyte- and neutrophil-predominant alopecias, they do not correlate with the clinical variants within these groups10.
In 2016, Fenning et al. performed an immunohistochemical analysis of three primary scarring lymphocytic alopecias (LPP, discoid lupus erythematosus [DLE], and CCCA) and they found that LPP and CCCA share identical CD123+ plasmacytoid cell (PDC) features, with PDCs arranged as single, interstitial cells, whereas DLE showed markedly different PDC characteristics, with PDCs comprising a greater percentage of the infiltrate and arranged in clusters11. These molecular findings suggested a common pathophysiology for LPP and CCCA, distinct from that of DLE.
In 2020, Jordan et al., published the largest comparative study of clinicopathological and immunophenotypic features of classic LPP and CCCA, where once more, they found no significance difference between the diseases, both composed of a CD4+ T-cell pre-dominant inflammatory infiltrate, lending further support to the identity of the two diseases2. The only subtle difference identified by the authors is that PDIRS in LPP is limited to inflamed follicles sparing noninflamed follicles, in distinction to CCCA, which has PDIRS in both inflamed and noninflamed follicles2.
Nevertheless, in the same year, Flamm et al. demonstrated that although CCCA is associated with a CD4-predominant T-cell infiltrate extending into the lower hair follicle, in contrast to both LPP and FFA that have CD8-predominant lymphocytic infiltrates12. According to the authors, evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from LPP and FFA. They also highlighted that affected follicles of CCCA had a higher number of CD1a+ Langerhans cells, as opposed to unaffected follicles, similarly to other forms of scarring alopecia, such as LPP, pointing to a similar destructive mechanism12.
Regarding these controversies, the precise histologic uniqueness of CCCA remains a dynamic and actively evolving area of interest2.
Management
Treatment of CCCA is notoriously challenging, and multiple therapies are often deployed, particularly in patients with advanced stages of hair loss.
Early intervention is paramount to delay and/or prevent follicular burnout.
One of the first-line therapies is behavioral modification. Although there is no consensus on specific hair styling recommendations due to conflicting data in the literature, it is advisable to avoid tension-inducing hairstyles and chemical relaxers6.
Standard medical treatment of CCCA depends upon the stage of disease. In the active phase, anti-inflammatory therapies involve high-potency topical corticosteroids or calcineurin inhibitors, intralesional corticosteroids, and oral tetracyclines (doxycycline or minocycline) or hydroxychloroquine13. Immunosuppressants such as mycophenolate mofetil and cyclosporine are therapeutic alternatives in patients with the difficult-to-control disease1. Topical 10% metformin and oral metformin formulations have also been prescribed based on the association of CCCA with metabolic syndrome6.
Once stabilized, maintenance is achieved with mid-potency topical corticosteroids and/or calcineurin inhibitors, when necessary13. Topical and oral minoxidil are used to improve hair coverage on the scalp, as they stimulate viable hair follicles, convert vellus to terminal hairs, and prolong the anagen phase of the hair cycle1.
Hair transplantation may be considered for stable disease, in which there is no active inflammation for at least 9-12 months1,6.
Recommended treatment of LPP is similar in many ways, including the anti-inflammatory and maintenance treatments. In addition, the effect of the 5-α reductase inhibitors (5 α RI), finasteride, and dutasteride, is better stablished, and authors state that it is the most beneficial therapeutic modality for LPP8,14. It is believed that these substances act on the etiology of the disease, with a probable hormonal mechanism8,14.
Discussion
CCCA remains a perplexing and understudied form of hair loss, particularly prevalent among individuals of African descent. Despite its prevalence and impact, there is ongoing debate regarding its etiology and classification.
One compelling perspective is to consider CCCA as a variant of LPP, a more extensively studied form of PCA, with several peculiarities ranging from clinical aspects to variations in therapeutic response. This viewpoint has garnered attention in recent years, shedding light on potential similarities and implications for treatment.
Supporting this theory are several key factors: similar age and gender prevalence (primarily young adult females), clinical presentation (one or multiple patches of alopecia, mainly on the vertex, which can be associated with symptoms such as trichodynia and itching, progressing to cicatricial alopecia), and trichoscopic findings (erythema and peripilar scaling, and loss of follicular openings). Some specific trichoscopic findings of CCCA are not found in LPP, such as a disrupted honeycomb pigmented network and irregularly distributed pinpoint white dots, but they are related to the normal trichoscopic differences in the scalp of dark-skinned patients. Notably, the degree of inflammation is significantly different, being milder in CCCA, which can be addressed either clinically or trichoscopically. However, the dark skin of CCCA patients may account for some of this difference, as it is more difficult to recognize inflammation in dark skin in both approaches.
Against this theory is the fact that CCCA only affects individuals of a specific ethnic background, African descent, suggesting it may be a distinct entity with a strong genetic basis. This is supported by evidence of an autosomal-dominant pattern of transmission and the identification of pathogenic genetic variants in the PADI3 gene. In addition, environmental triggers, including cultural hair grooming practices, play a significant role in the development of CCCA.
Distinguishing these two entities histologically and immunohistochemically remains a major topic of research. Both present perifollicular lymphocytic infiltrates, identical CD123+ plasmacytoid dendritic cell features, and ultimately fibrosis. It has been consistently demonstrated that the inflammatory infiltrate in CCCA is predominantly composed of CD4+ T-cells. However, there is some controversy regarding whether LPP is also mainly composed of CD4+ cells or, in contrast, CD8+ cells12. In addition, CCCA uniquely exhibits PDIRS in both inflamed and non-inflamed follicles, a feature not typically seen in LPP12. However, despite these minor distinctions, the histologic and immunophenotypic similarities are so significant that LPP and CCCA could be considered the same disease.
The currently recommended treatments for CCCA and LPP are similar. However, the roles of finasteride and dutasteride are better established in LPP and warrant further study in CCCA patients.
Considering all of these, we even hypothesize that instead of considering CCCA as a subtype of LPP, it may be more accurate to view lymphocytic PCAs as a continuous phenotypic spectrum, including FAPD, CCCA, and LPP. Supporting this we have the observation that some patients with CCCA also present overlapping trichoscopic findings with AGA, such as miniaturization, resembling FAPD. In our viewpoint, genetics (race, gender, and specific genes) may drive the pathology, but environmental factors may expose different epitopes to the immune system, leading to slightly different clinical, trichoscopic, and pathological presentation and evolutions.
Conclusion
Further research is needed to elucidate the precise relationship between these conditions, which may be reflected in an updated classification system for lymphocytic PCAs in the future. These advances will help to develop targeted therapeutic strategies, potentially improving outcomes for affected patients.