Introduction
Vitiligo is the most common pigmentation disorder in humans, with a prevalence of 0.5-2% in the world population and occurs due to a selective destruction of melanocytes. Vitiligo is characterized by areas of achromic, non-squamous skin with well-defined borders, it occurs itself in all age groups, although most cases start before the age of 20, with no significant differences between gender and ethnicity1,2.
Various theories have been proposed to explain the decrease in melanocytes, but it is now well established that it is a multifactorial, autoimmune disease associated with genetic, environmental and metabolic factors, oxidative stress, and abnormalities in cell adhesion. The “convergence theory” or “integrated theory” suggests that the correlation of all the proposed mechanisms contributes to the destruction of melanocytes1,2.
Genome-wide association studies (GWAS) and allelic association studies have identified approximately 50 susceptibility loci associated with vitiligo, several shared with autoimmune diseases, mainly thyroid diseases, pernicious anemia, diabetes mellitus, alopecia areata, systemic lupus erythematosus, rheumatoid arthritis, scleroderma, dermatomyositis, Sjögren’s syndrome, Addison’s disease, autoimmune gastritis, inflammatory bowel disease, psoriasis, atopic dermatitis, ocular, and hearing system abnormalities1,3,4.
Leprosy is still a major public health challenge, especially in developing countries like Brazil, which has the second highest prevalence in the world. It is a chronic infectious disease caused by the acid-fast bacillus Mycobacterium leprae and mainly affects the mucosa of the upper respiratory tract, eyes, skin, and peripheral nerves. In some cases, sensory and motor impairment leads to deformities and disabilities5.
It also has a multifactorial pathophysiology related to genetic, immunological, and environmental aspects, which determine an individual’s susceptibility to the bacillus. The sequencing of the M. leprae genome in different population revealed low variability with 99.995% of the genetic material identical in the different samples studied, which suggests that the variability of the clinical manifestations of the disease is mainly due to intrinsic host factors. The M. leprae genome contains a low percentage of functional genes with a high percentage of pseudogenes and non-coding genes6.
The clinical spectrum of leprosy with its polar forms, tuberculoid and virchowian, represents the response of polarized T cells, Th1 and Th2. However, the predominance of one response does not exclude the presence of the other5,7.
Multibacillary (MB) individuals generally develop a cellular immune response unable to contain bacillary proliferation, with an intense humoral response and high titters of specific antibodies against the mycobacteria. Genes involved in the humoral immune response, mainly genes for immunoglobulin receptors or proteins of the classical complement pathway, are highly expressed in virchowian and borderline MB leprosy and in leprosy reactions6.
There are seven articles in the world literature that correlate the two diseases, leprosy and vitiligo. In 1978, Jopling observed a significant prevalence of 7% of cases of vitiligo in patients with lepromatous leprosy and no cases among a significant number of patients with tuberculoid leprosy (TT); at the time, he investigated a possible autoimmune cause for vitiligo, since a variety of autoantibodies were identified in the lepromatous pole, such as antinuclear antibodies, antithyroid antibodies, antisperm antibodies, and rheumatoid factor8,9.
A retrospective study identified a high prevalence of vitiligo in patients with lepromatous leprosy (10.9%) compared to the general population (0.34%) with high significance (p < 0.0001). The study was carried out in the only reference hospital for the follow-up of leprosy patients in Martinique, a place with a highly endemic leprosy. No patient from the tuberculoid pole had vitiligo10.
Pavithran published an article in 1991 on vitiligo in patients with lepromatous leprosy followed up in a reference hospital in India. Among 400 patients with lepromatous leprosy seen by the author over 12 years, this co-morbidity was found in 12% of the patients contrasting with only 2% among 1,600 tuberculoid patients seen in the same period. 4000 healthy volunteers were examined, representing the general population in this area, and a 2.2% occurrence of vitiligo was observed. The study indicated that the prevalence of vitiligo is significantly higher in patients with lepromatous leprosy than in the general population and in patients with TT11.
Objectives
In the historical casuistry of the leprosy outpatient clinic of the dermatology department of a highly complex hospital in the city of Rio de Janeiro, vitiligo was diagnosed in some patients of the MB pole, but similarly to what is reported in the literature, in no cases of the paucibacillary pole. Based on this finding, we began a literature review on the correlation between the two diseases and their pathophysiological mechanisms.
Materials and methods
The leprosy outpatient clinic is a reference for diagnostic elucidation, treatment of therapeutic complications, and surgical rehabilitation in leprosy. Among all our cases, we looked retrospectively for the association with vitiligo in medical records from 1999 to 2022. Patients with vitiligo were also examined and followed up at the vitiligo outpatient clinic and some cases underwent skin biopsy to document the diagnosis.
We analyzed demographic data and clinical data both from vitiligo (non-segmental) and leprosy, namely, concerning duration of disease and therapy performed.
Results
Five representative cases of vitiligo in leprosy patients were identified (3M/2F) aged 42 (mean). They all suffered from MB bacterial vaginosis patients, all of them with reactions (before, during, and after multi-drug treatment). All cases were adequately treated with multidrug therapy. Reactions were treated with thalidomide and oral corticosteroids.
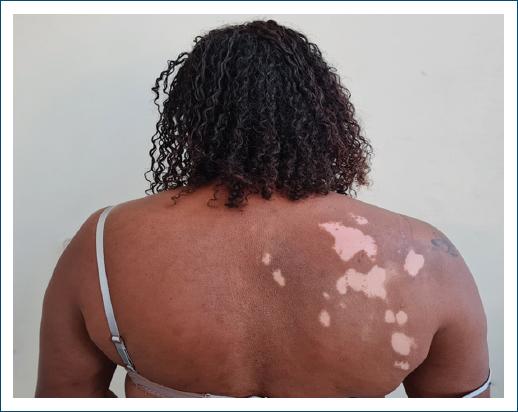
Vitiligo lesions appeared on average 10 years after the diagnosis of leprosy, in two patients during the follow-up of leprosy sequelae, and in one, the onset of the first lesions was observed in the outpatient clinic (Figs. 1 and 2). In two cases, they who underwent skin biopsy histopathology showed attenuation of basal melanic pigmentation, with no presence of acid-fast bacilli. Gender, age, location of the lesions, onset of vitiligo, presence of leprosy reaction, comorbidities, and treatment for leprosy were also studied, as shown in table 1.
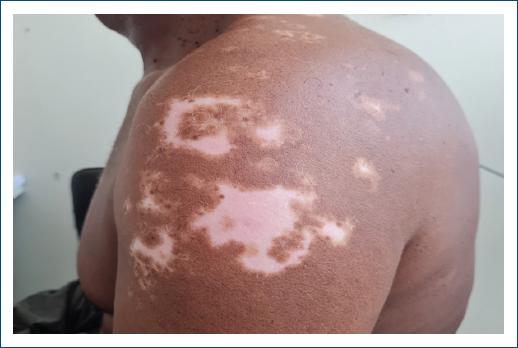
Figure 2 Vitiligo lesions with repigmentation in a patient previously diagnosed with multibacillary leprosy.
Table 1 Clinical characteristics of the patients studied
| Patients | Age | Gender | Year of leprosy diagnosis | Leprosy type | Reaction type | Year of vitiligo diagnosis | Location of vitiligo lesions | Skin biopsy | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | 1999 | BL | Type I e II | 2014 | Face, back and extension of upper and lower limbs | Yes | MDT-24 months |
| 2 | 43 | M | 2000 | BL | Type II | 2015 | Single lip lesions | No | MDT-24 months |
| 3 | 42 | F | 2001 | BL | Type II | 2008 | Gluteal region | Yes | MDT-24 months |
| 4 | 37 | M | 1999 | BL | Type I | 2013 | Elbows and gluteal regions | No | MDT-12 months |
| 5 | 38 | F | 2010 | BL | Type II | 2020 | Right anterior and left posterior thorax | Yes | MDT-24 months |
BL: borderline lepromatous leprosy; MDT: multi-drug therapy.
Vitiligo remained stable in two patients followed and varied in more extensive cases for approximately 1 year, when stabilization of the lesions was observed. Two patients still remained under follow-up with repigmentation of the lesions. Treatment with high-potency topical corticosteroids and tacrolimus was performed.
Discussion
The experience of this service corroborates the higher occurrence of vitiligo in patients in the MB pole, as reported in the world literature, particularly the articles by Jopling and Boisseau-Garsaud et al. with no cases in the paucibacillary pole. Although the study by Pavithran found cases in the paucibacillary pole, the prevalence was lower than that found in the general population and the MB pole9-12.
Vitiligo lesions of the mutibacillary pole were well differentiated from the hypochromic lesions with ill-defined borders that can occur in the tuberculoid pole in all the studies. The pathophysiological process that triggers vitiligo in leprosy patients has not yet been established. It is known that the two diseases with complex immunogenetic mechanisms can lead to melanocyte loss by different routes. Autoimmune disorders are correlated with evidence of circulating autoantibodies in both conditions9-11,13.
Leprosy is a complex disease which, in addition to the circulation of bacilli in an endemic environment, depends essentially on the host’s own factors to establish effective innate and adaptive immunity to M. leprae. However, many immunogenetic mechanisms have yet to be fully elucidated and represent a major scientific challenge. Its characteristic spectrum results in clinical and immunological polarity in which TT is characterized by strong pro- and inflammatory immunity, mediated by T helper-1 (Th1) and Th17, with efficient control of bacterial infection. And at the opposite end of the spectrum, virchowian leprosy with a T helper-2 (Th2) response, where there is intense multiplication of M. leprae with functional deficiency of Mycobacterium-specific T cells, but high titers of specific antibodies and an increase in regulatory T cells, as well as greater action by B cells, producing anti-inflammatory cytokines that inhibit the microbicidal function of macrophages and facilitate the spread of the disease14-17.
Between the poles of the clinical spectrum, there are unstable borderline states with a mixed immunological profile, which represent the majority of leprosy patients and are more prone to leprosy reactions. These are two distinct complications with acute and exacerbated inflammatory episodes, which can arise spontaneously before, during, and after treatment; both reactions are rarely present in the same patient. The reverse reaction or Type 1 reaction (T1R) of local delayed hypersensitivity, which occurs in the tuberculoid pole, is characterized by infiltrations of TCD4+ cells. And in erythema nodosum leprosum or type 2 reaction (T2R), there is deposition of immune complexes in the bloodstream and tissues. All patients in this study developed the virchowian dimorphic form and evolved with reactions; one patient had both forms of reaction, a rarer condition in leprosy14-16.
Cytokines play an important role in the entire immunological spectrum of leprosy, with specific profiles. In the lepromatous pole, there is an increase in interleukin (IL-4), IL-5, IL-10, and transforming growth factor-beta 1 (TGF-β1) in the lesions, which inactivate the microbicidal function of the macrophages and facilitate bacillary proliferation. The tuberculoid pole is mediated by CD4+ with increased interferon (IFN)-gamma. High levels of tumor necrosis factor (TNF)-alfa and IFN-alfa have been observed in all forms of leprosy and in leprosy reactions18. The balance of the response between Th1 and Th 2 lymphocytes is maintained by CD4 + CD25 + FoxP3 + Tregs producing IL-10 and secreting TGF-β, which shows increased expression in the virchowian pole, responsible for the anergic picture5,17,19.
B cells are also present in immunopathogenesis with an immunosuppressive function. The cytokines secreted by various immune cells act to control the intensity of the host’s immune response or promote regeneration in the organism, even acting to convert functionally antagonistic cells5,20.
In T1R there can be a transition from a Th2 to a Th1 profile with increased production of IFN-γ and CXCL10. Cytokines such as IL-2, INF-γ and TNF-α, TGF-β, IL-17, and IL-23 and Treg act in T1R. Cytokines IL-1β, IL-4, IL-6, IL-10, and TNF-α are increased in T2R21. In T2R, there is also C3 consumption, similar to diseases such as lupus and acute glomerulonephritis. A Brazilian study showed that all the patients surveyed with C4B deficiency developed T2R. 6 It has been discussed in the literature whether the activation of signal transducer and activator of transcription 1 (STAT 1) by IL-10 under the action of IFN-α, inducing the synthesis of chemokine 9 (CXCL9) and chemokine 10 (CXCL10) could also be applied to leprosy19,22.
Vitiligo also has a complex pathophysiology in which different pathogenetic mechanisms can be associated, leading to the same characteristic clinical result. It is known that the cause of depigmentation is the loss of melanocytes from the skin, but what can trigger this loss has always been questioned23,24.
Recent studies have shown that melanocytes are important neuroimmune cells that work together with keratinocytes, fibroblasts, and Langherans cells to control the infectious and inflammatory condition of the skin, contributing to the process of phagocytosis of invading pathogens, acting as antigen-presenting cells and they enter into apoptosis when infected. Melanocytes produce important chemokines and cytokines, such as IL-1β, IL6, and TNF-α, and when stimulated by cytokines, they express several important marker proteins, such as intercellular adhesion molecule-1, vascular cell adhesion molecule-11, CD40, and human leukocyte antigen (HLA) classes I and II, which are essential for T-cell co-stimulation. Cytokines such as IFN-γ, hormones such as α-melanocyte-stimulating hormone and factors such as TGF-β are involved in the control of skin pigmentation. IFN-γ is secreted by immune cells recruited in infectious and inflammatory processes. Keratinocytes, fibroblasts, and T-cell infiltrates modulate the immunobiology and pigmentation of melanocytes. Melanin can contribute to the death of pathogens by limiting the absorption of nutrients and by the cytotoxic activity of melanogenic precursors and reactive oxygen species generated during melanogenesis23,25,26.
According to the literature, the convergence theory is the most widely accepted to explain the pathophysiological mechanism of vitiligo, in which the loss of pigment occurs by different mechanisms, with a consensus on its autoimmune and multifactorial nature in which oxidative stress would be the initial trigger for the destruction of melanocytes, which in patients with vitiligo have decreased adhesiveness to keratinocytes and greater susceptibility to oxidative stress1,23,27. It is currently believed that environmental stress associated with genetic susceptibility compromises the antioxidant system. Mitochondria are the main inducers of reactive oxygen species (ROS) and their functionality is altered in vitiligo. The increase in ROS activates the secretion of exosomes by melanocytes. Exosomes contain melanocyte-specific antigens, heat shock proteins, miRNAs, and damage-associated molecular patterns, which deliver vitiligo target antigens to adjacent dendritic cells, promoting their activation and differentiation1,28,29. At the same time, CD8+ T cells in vitiligo lesions produce various cytokines such as IFN-γ, which, by binding to their own receptor, activate the Janus kinase pathway, JAK-STAT (JAK1, JAK2, and STAT 1) and lead to the secretion of the chemokines CXCL9 and CXCL10 into the skin. These chemokines have the same receptor, CXCR3 on CD8+ T cells. CXCL9 promotes the mass recruitment of these cells, while CXCL10 promotes their effector function and their localization within the epidermis, which increases the inflammatory process1,29,30.
In addition to the pathophysiological mechanisms mentioned above, GWAS are also important because they identify genetic variants associated with complex diseases and in dermatology more than 40 GWAS have been identified in some diseases, including vitiligo and leprosy. In the two diseases studied, several susceptibility genes related to humoral, innate, and adaptive immunity were observed, such as the HLA system, toll-like receptors, cytokines (ILs, INF, TNF), and chemokines. Common genetic variants associated with the risk of vitiligo are involved in immunoregulation, apoptosis, and melanocyte biology4,31,32.
Conclusion
Leprosy and vitiligo have already been established as complex diseases due to their pathophysiological characteristics, which are still highly stigmatized by society and whose immunogenetic mechanisms are under investigation.
There are few reports on the association between the two diseases in the world literature. However, all existing studies to date have shown a predominance of vitiligo in the MB pole.
Humoral, innate, and adaptive immunity play an important role in the pathophysiology of the MB pole, leprosy reactions, and vitiligo, but they have not yet been fully elucidated. GWAS have revealed several susceptibility genes that also need to be better understood.
As reviewed in this paper, immunological alterations can lead to the concomitance of both conditions. Both the initial infectious event and the entire immunological cascade triggered in the MB pole, as well as the immunological exacerbations of the reactions, are stressful events that involve many cytokines. However, there is currently no precise idea of the factors that define the development of vitiligo or the selectivity for the MB pole.