INTRODUCTION
Peritoneal dialysis (PD) is an effective kidney replacement therapy in managing patients with end‑stage kidney disease.1 There are several clear advantages offered by PD therapy in terms of preservation of residual kidney function, patient satisfaction, and the promotion of an optimal quality of life.2 Although variable factors influence the success of PD therapy, including a well‑functioning peritoneal catheter, adequate peritoneal membrane and good patient adherence to therapy, nephrologists are often plagued by the presence of a history of abdominal surgery.2‑4
The success of PD depends critically on the structural and functional integrity of the abdominal cavity and the peritoneal membrane. Uncorrectable discontinuities of the former and irreversible damage to the latter represent formal contraindications for this technique.1 Intestinal resection, peritoneal adhesions, or membrane fibrosis are occasionally present before PD is started, often due to surgical procedures and/or inflammatory (most commonly infectious) injury. It is estimated that the selection of PD is dismissed in 6%-38% of potential candidates for these reasons.5,6A history of catastrophic or recurrent abdominal events or the presence of radiologically evident distortions of the abdominal anatomy is helpful to presume the impracticability of PD, but in many other cases, the feasibility of the technique can only be ascertained after a catheter is inserted and treatment is attempted.7
To our knowledge, a limited number of studies have examined the relationships between clinical outcomes, complications, and PD technique survival with the history of previous abdominal surgery.8‑10We have performed a retrospective, observational study to disclose the effect of prior abdominal surgical procedures on PD technique survival, dialytic efficacy and risk of peritonitis.
METHODS
We performed a single centre, retrospective study, including 155 peritoneal dialysis patients followed at our unit, from September 2017 to September 2022. We included all patients in peritoneal dialysis for at least three months, with regular follow‑up in our centre. Experienced surgeons in our institution performed all the PD catheter insertions. Catheter implantations were performed using a laparoscopic approach or a mini‑laparotomy. Laparoscopic approaches are used in patients with a previous history of major abdominal surgeries. Demographic and clinical characteristics, such as age, gender, chronic kidney disease etiology, comorbidities, time on PD, dialytic efficacy at baseline (Kt/V in the first 3‑6 months of PD), peritoneal equilibration test (PET) results and episodes of peritonitis were registered from our unit´s database. Afterwards, the patients were divided into 2 groups according to previous abdominal surgery status and correlation with other variables was analysed. We further categorized the abdominal procedures according to the magnitude of peritoneal aggression: major abdominal surgeries included open and/or complicated appendicectomy or cholecystectomy; pancreatic or splenic surgery; limited gastrointestinal resection; exploratory laparotomy; transperitoneal vascular surgery and transperitoneal urologic or gynecological procedures. The primary outcome was technique survival, defined as the time to transition to hemodialysis. Secondary outcomes were dialytic efficacy at baseline and risk of peritonitis.
We used the Pearson chisquare test to compare groups regarding qualitative variables and the Student t‑test or the Mann‑Whitney‑U test for quantitative variables. Risk factors for the occurrence of peritonitis were identified using univariate and multivariate logistic regression. Univariate analysis for technique survival was performed using the Log Rank test for qualitative variables and Cox proportional hazards for quantitative variables. Variables with a p-value ≤ 0.1 in univariate analysis were included in Cox proportional‑hazards regression models to assess for confounding effects. A p ‑value < 0.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS Statistics version 28 software (IBM, Armonk, NY, USA).
RESULTS
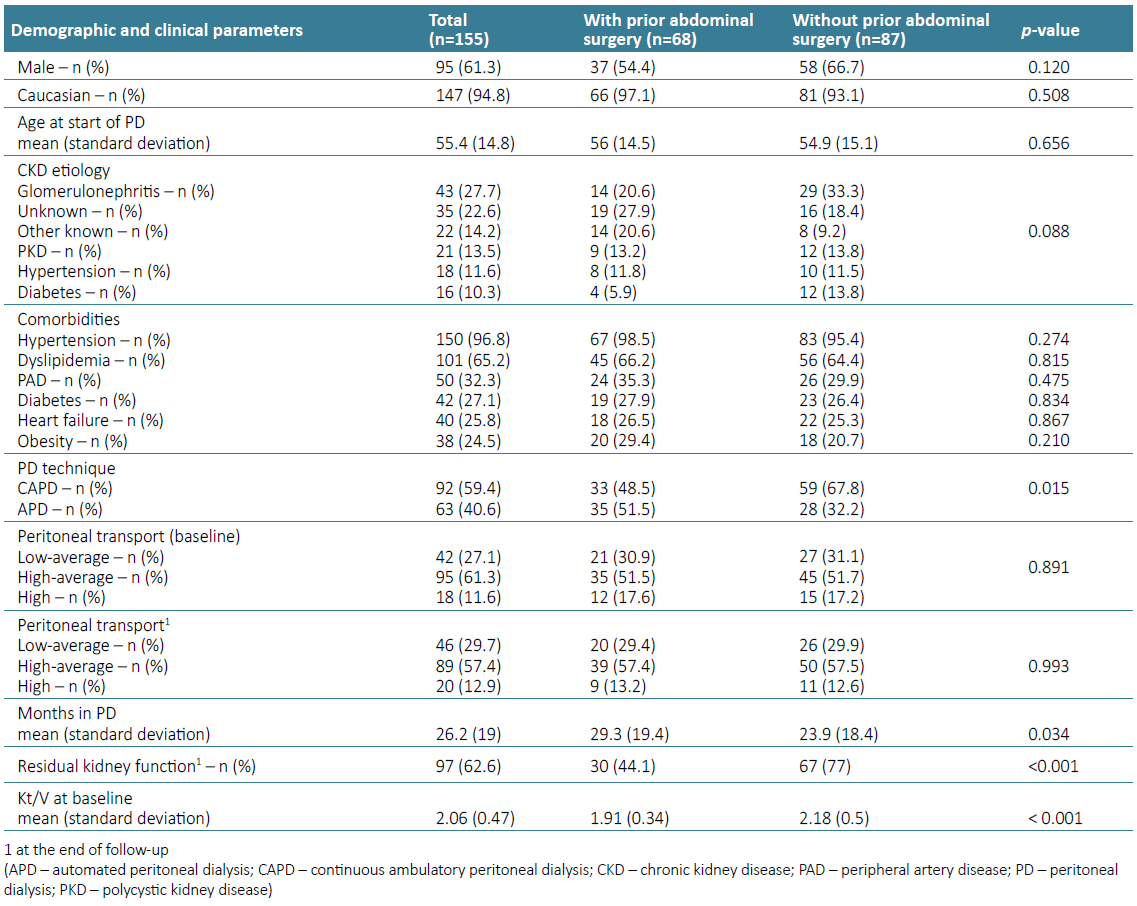
Our sample was predominantly male (61.3%), Caucasian (94.8%), and started PD with an average age of 55.4 years. The most known cause of CKD was chronic glomerulonephritis (27.7%), followed by polycystic kidney disease (13.5%). The main comorbidities were hypertension (96.8%), dyslipidemia (65.2%), and peripheral arterial disease (32.3%). During the follow ‑up period, patients stayed in PD for an average of 26.2 months. The demographic and clinical characterization of the study sample is detailed in Table 1.
Considering the patient’s abdominal surgical history, both groups did not present statistically significant differences regarding their gender, comorbidities’ prevalence, CKD etiology, and age distribution (Table 1). As expected, the prevalence of APD was higher in patients with previous abdominal surgery (p‑value=0.015).
Regarding abdominal surgery status, 43.9% (n=68) had a history of abdominal surgery before PD catheter placement, of whom 36.8% (n=25) were found to have peritoneal adhesions during the catheter implantation procedure; most (88%, n=22) underwent adhesion lysis at the same surgical time. Twenty‑two percent of the patients (n=15) with a history of abdominal surgery had major procedures: four nephrectomies, three cesarians, three hysterectomies with bilateral salpingectomy, two radical prostatectomies, two open appendicectomies and one complicated cholecystectomy. Of the patients with previous abdominal surgery, a total of 69.1% (n=47) had a history of hernias, and their correction occurred in 27.7 (n=13) patients prior to the start of the PD technique. There was no statistically significant difference in the prevalence of intra-abdominal adhesions between patients with major and minor abdominal procedures (37.7% vs 33.3%, p‑value=0.755).
During the follow‑up period, 42 patients transitioned to hemodialysis (120 per 1000 patient years), 36 received a kidney transplant (110 per 1000 patient‑years) and 13 died (40 per 1000 patient‑years). Main cause of death was infectious conditions (n= 6; 46.2%), followed by cardiovascular causes (n= 4; 30.8%). The transition to hemodialysis was related to episodes of peritonitis (n= 22; 52.4%), ultrafiltration failure (n= 13; 30.9%), provider comfort (n= 5; 11.9%) and catheter ‑related problems (n= 2; 4.8%). Sixty-six patients developed peritonitis during follow‑up, with an overall peritonitis rate of 0.35 episodes per patient year.
Technique Survival
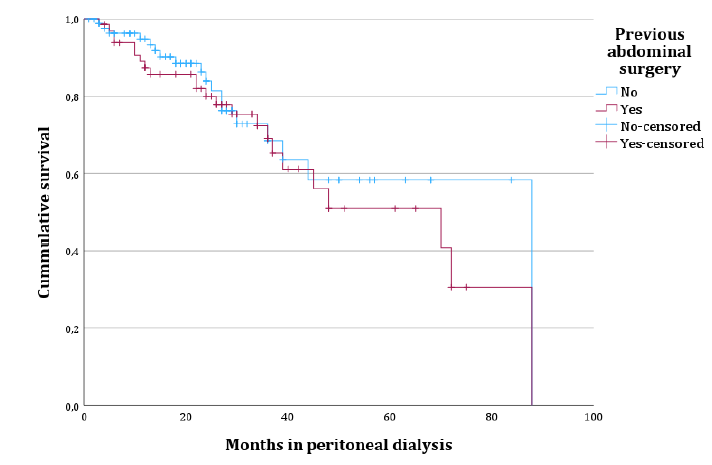
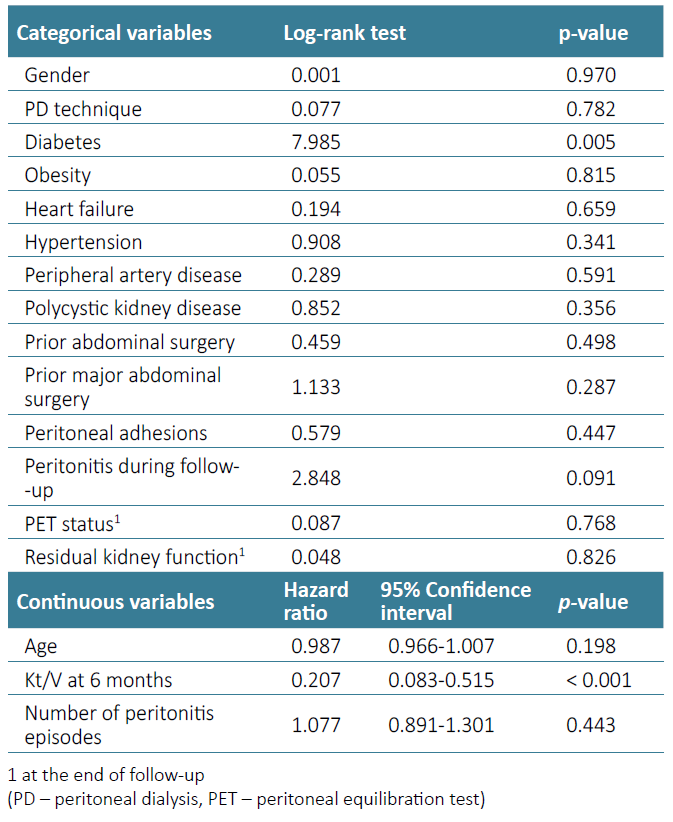
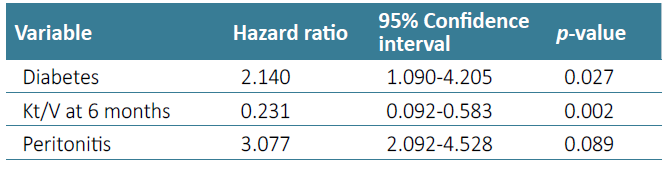
Considering technique survival, history of previous abdominal surgery (Log rank 0.459, p-value=0.498) [Fig. 1] or the finding of peritoneal adhesions at PD catheter placement (Logrank 0.579, p-value=0.447) were not significantly associated with detrimental technique survival in univariate analysis, which is detailed in Table 2. History of major abdominal surgery also did not impact technique survival (p-value=0.287). Diagnosis of peritonitis during follow‑up (p-value=0.091), diabetes (p-value=0.005) and baseline Kt/V values (p-value < 0.001) were included in a multivariate model to assess for confounding effect. In a Cox regression model, diabetes (hazard ratio 2.140, p‑value=0.027) and baseline Kt/V values (hazard ratio 0.207, p -value < 0.001) were the only independent prognostic variables in our sample (Table 3).
Baseline and 12 -Month Kt/V Values
Despite having no impact on technique survival, a history of prior abdominal surgery was associated with a lower baseline Kt/V (1.91 vs 2.18, p-value < 0.001). At 12 months, Kt/V remains similar in both groups (1.88 vs 2.12, p-value < 0.001). The finding of intra‑abdominal adhesions at catheter implantation was also associated with a lower baseline Kt/V (1.94 vs 2.09, p -value=0.153), although it did not meet statistical significance. Patients with diabetes (1.92 vs 2.12, p -value=0.019) or heart failure (1.92 vs 2.11, p -value=0.025) also presented statistically significant lower baseline Kt/V values. Baseline Kt/V values did not significantly differ between patients with major or minor abdominal procedures (p -value=0.07).
Risk of Peritonitis
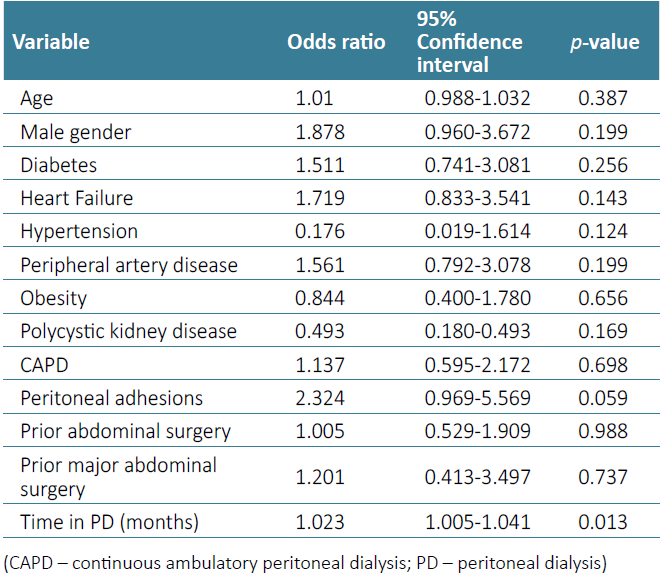
In our study sample, prior abdominal surgery did not influence the risk of peritonitis (OR 1.005, p-value=0.988), or the average number of peritonitis episodes during follow‑up (0.87 in the abdominal surgery group vs 0.58 in the control group, p-value=0.649). Patients with previous major abdominal procedures also did not present a higher risk of peritonitis (OR 1.201, p‑value=0.737). The results of univariate logistic regression analysis for other variables are detailed in Table 4.
However, the finding of intra‑abdominal adhesions at PD catheter implantation was associated with a higher risk of peritonitis (OR 2.324, p-value=0.059) and a higher average number of peritonitis episodes during follow‑up (1.4 vs 0.64, p‑value=0.012). When adjusting for other relevant variables in multivariate logistic regression, namely gender, time in PD, peripheral artery disease, diabetes, and heart failure, only time in PD (OR 1.024, 95% CI p-value=0.018) and peritoneal adhesions (OR 1.825, p‑value=0.036) presented a statistically significant association with peritonitis risk. The area under the ROC curve for our model was 0.681.
DISCUSSION
Peritoneal dialysis (PD) is an excellent treatment option for patients with end‑stage kidney disease and has been shown to improve patient satisfaction with a favorable cost‑utility ratio. Many surgeons and physicians believe that patients with prior abdominal surgeries or other abdominal complications are not viable candidates for PD.10 The option of PD is often discouraged in patients with this kind of background,2‑6 although this therapy has proven to be feasible even after seemingly aggressive abdominal procedures, including limited intestinal resections.2 Some reports have shown a high prevalence of intra‑abdominal adhesions in PD patients with prior abdominal surgeries and a detrimental effect of adhesions on PD adequacy, without an apparent effect on technique survival.10Indeed, our results have shown a similar reality, in which around 37% of patients (n=25) with prior abdominal surgical procedures presented peritoneal adhesions at PD catheter placement, with a detrimental impact on baseline PD adequacy, but not in technique survival. Despite its impact on baseline PD adequacy, it is worth noting that both groups presented average Kt/V values above the 1.7 threshold. Although there is a statistically significant difference, it may not be clinically relevant. We can hypothesize that the use of smaller dwell volumes in patients with prior abdominal surgical procedures might be responsible for these results, without impacting overall dialysis adequacy and clinical outcomes, such as technique survival. The impact of Kt/V values should be carefully interpreted, since small differences in Kt/V might provide statistical significance but have no clinical impact when considering overall dialysis adequacy. Lower weekly Kt/V baseline values might be a surrogate of lower residual kidney function at the start of PD, which has a recognized prognostic impact on technique survival.11
Prior abdominal surgical procedures (major or minor) did not have a detrimental impact on the risk of peritonitis, with similar peritonitis incidence rates between the two groups. However, patients with peritoneal adhesions presented a trend for increased peritonitis risk, even when adjusting for time in dialysis or other relevant comorbidities. Peritoneal adhesions might act as a surrogate of abdominal injury, correlating with acute/chronic inflammation and injury to the peritoneal membrane, which undergoes progressive fibrosis, angiogenesis and vasculopathy. Disruption of the monolayer of mesothelial cells, with induction of mesothelial to mesenchymal transition in response to pro‑inflammatory/pro-fibrotic stimuli, might impair the peritoneum’s first line of defense against microorganisms.12 Furthermore, intra‑abdominal adhesions increase the risk of small‑bowel obstruction which may favor transmigration of bowel flora and increased peritonitis risk.13
However, our results should be interpreted with caution. We must highlight an evident limitation of our study that is related to the disproportionate number of patients with and without peritoneal adhesions. This disproportion of patients limits comparisons between groups and the discrimination capacity of our model.
Our findings are consistent with the notion that minor surgical procedures are not harmful to the peritoneal membrane or that the latter has a significant capacity to recover from this type of injury. Our study suffers some significant limitations, including a single‑center, retrospective design. The study population was relatively small, resulting in a shortage of statistical power in subgroup analysis.
Our study shows that this group of patients deserves a more detailed evaluation before starting PD to understand if they are good candidates for the technique. When appropriately planned, PD can still be an acceptable option for patients with end‑stage kidney disease and certain abdominal complications, including previous abdominal surgery. Anticipating complications and changing the PD prescription accordingly can allow such patients to initiate PD without interruption, thus maintaining their lifestyle and avoiding increased medical expenses.