INTRODUCTION
Kidney transplant is the treatment of choice to end‑stage renal disease (ESRD), improving patient´s morbidity and mortality compared with other renal replacement therapies (RRT) such as hemodialysis.1 Advances in immunosuppressive therapies have substantially reduced the incidence of acute rejection in the past three decades. Acute rejection rates have steadily declined from nearly 100% in the first half of the 20th century to approximately 10% more recently.2 Acute rejection remains, however, one of the main causes of graft loss. In fact, understanding the factors affecting graft rejection can help to estimate the probability of immunologic graft damage, and accurately identify the type and severity of acute rejection, while guiding appropriate treatment and promoting graft survival. Multiple factors have been proposed in the literature, including pre-surgery factors related to donor and recipient characteristics such as age, sex, race, HLA mismatches and preformed donor specific antibodies (DSA),3,4 peri‑operative parameters such as cold and warm ischemia times,5,6and post‑operative immunosuppressive treatment.7 Tissue biopsy remains the gold standard for evaluating immunologic graft damage. Histology is interpreted using the Banff classification of kidney allograft pathology, which has undergone extensive updating and revision since its development in the 1990s. The diagnostic criteria for T cell-mediated rejection have undergone little change in recent years, and include lymphocytic infiltrate of tubules (tubulitis) and larger vessels (vasculitis), with the severity of these lesions depending on the degree of lymphocytic infiltrate per high‑powered field.8 The aim of our study was to determine the incidence and assess risk factors for acute T‑cell mediated rejection (TCMR) and borderline rejection diagnosed in early protocol kidney transplant biopsies.
MATERIAL AND METHODS
We conducted a single ‑center retrospective study of kidney transplant recipients between January 2021 and June 2022 at the Transplantation Unit of Hospital Curry Cabral, Portugal. Kidney recipients whose grafts were lost due to surgical complications and those who are concurrently receiving non ‑renal solid organ transplantation were excluded. Living donor transplants were included. Patients who received a second renal graft, with 5 or 6 HLA mismatches, PRA‑CDC 25%, HIV infection, African American patients, preformed DSA or donor after cardiac death were treated with thymoglobulin intravenous (IV) 1.5 mg/kg for 7 days as induction therapy. Those with PRA ‑CDC >50% also received 2 g/kg IV immunoglobulin (IVIG). Other patients were treated with basiliximab two doses of 20 mg IV, on days 0 and 4 post‑surgery. All patients received 3 pulses of 500 mg IV methylprednisolone. Maintenance immunosuppression regimens were similar in all patients and included tacrolimus (TAC), mycophenolate mofetil (MMF) and prednisolone.
All patients underwent protocol kidney biopsy during the first 2 weeks after transplantation. Rejections were classified as subclinical in those without delayed graft function requiring HD during the same period. Histological changes were evaluated and graded based on Banff classification 2019.8 According to biopsy results, patients were classified into two groups: patients with TCMR or borderline rejection, and those without rejection. Clinical and demographic characteristics including recipient age and sex, donor age and sex, donor cause of death, etiology of ESRD, HLA mismatching, preformed HLA DSA, warm and cold ischemia times and type of immunosuppression were collected and compared among groups. Statistical analysis was performed using SPSS version 23.0 for Mac OS X®. Continuous variables were presented as mean and standard deviation or median and interquartile range (IQR) for variables with skewed distributions. Nominal variables were presented as number (frequency) and percentage. A p value < 0.05 was considered statistically significant. Clinical characteristics were compared among groups using the Student T-test for normally distributed continuous variables, Mann‑Whitney U‑test for skewed distributed continuous variables and Chi‑square test for categorical variables. Univariate and multivariate analysis were performed to identify predictors of TCMR and borderline rejection in our population by logistic regression (p<0.1 was considered as the inclusion criterion for factors that could be added into multivariate analysis).
RESULTS
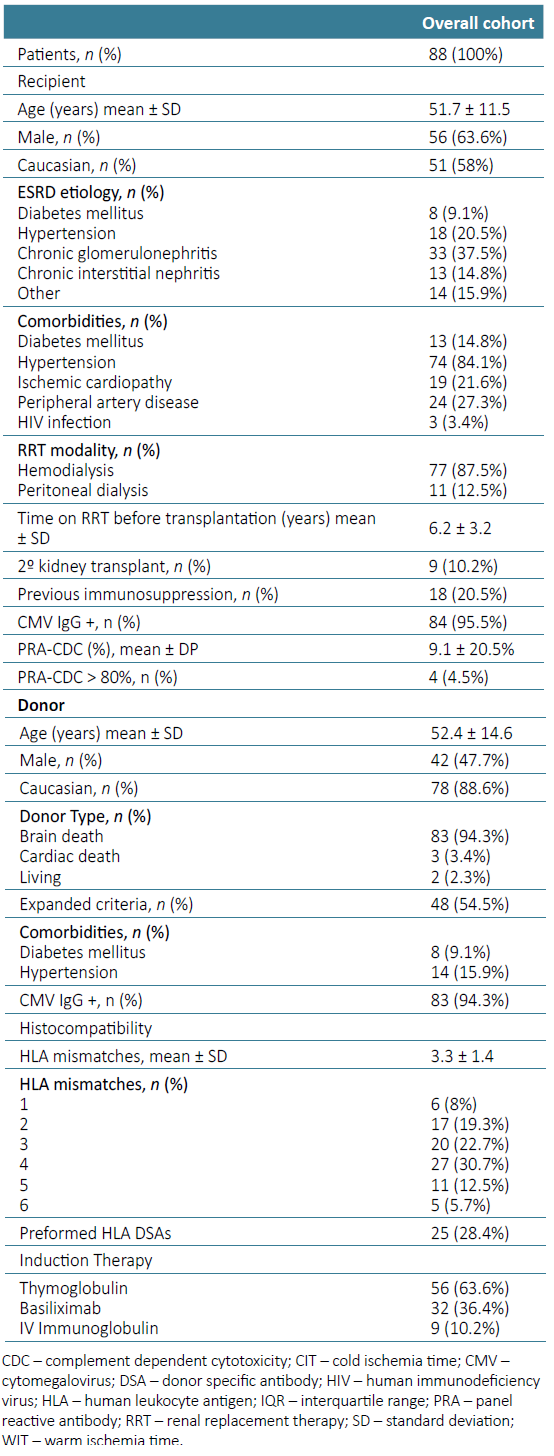
There were 88 patients submitted to kidney transplant during this period. Mean age was 51.7 ± 11.5 years, 56 (63.6%) were male, 51 (58%) were Caucasian, 13 (14.8%) were diabetic and 74 (84.1%) had arterial hypertension. Nine (10.7%) patients received a second graft and 17 (19.3%) were previously treated with immunosuppression. Mean CDC‑PRA was 9.1% ± 20.5%. Four patients (4.5%) were highly sensitized (CDC‑PRA >80%). Two (2.3%) donors were living donors, 83 (94.3%) brain‑dead donors and 3 (3.4%) donors after cardiac dead Maastricht II. Mean donor age was 52.4 ± 14.6 years and mean HLA mismatches 3.3 ± 1.4. Seven patients (8 %) had 1 HLA mismatch, 17 (19.3%) had 2, 20 (22.7%) had 3, 27 (30.7%) had 4, 11 (12.5%) had 5, and 5 (5.7%) patients had 6 HLA mismatches. Twenty‑five (28.4%) patients had pre-formed DSA. The induction immunosuppression included thymoglobulin in 56 (63.6%) patients and basiliximab in 32 (36.4%). Nine patients with PRA‑CDC > 50% or pre‑formed DSA were treated additionally with IV immunoglobulin (Table 1). Mean cold ischemia time (CIT) was 11.2 ± 5.1 hours and warm ischemia time (WIT) 27.6 ± 7.4 minutes. Twenty‑five (28.4%) patients presented delayed graft function and required at least one HD session in the first week after transplantation.
Fourteen patients (15.9%) had acute rejection on early protocol biopsy: 3 borderline rejection, 3 TCMR IA, 5 IB, 1 IIA and 2 IIB. Among those, 10 patients (71.4%) had subclinical rejection and 4 delayed graft function requiring HD. There were no complications related to renal graft biopsies in our cohort.
Patients with borderline and TCMR IA were treated with 3 pulses of 500 mg IV methylprednisolone. Those with TCMR IB, IIA, and IIB received 3 pulses of 500 mg IV methylprednisolone plus 1.5 mg/kg IV thymoglobulin for 3 to 7 days.
Clinical and demographic characteristics including donor and recipient age, sex, race and comorbidities, etiology of ESRD and time on renal replacement therapy before transplantation were similar in the two groups. A significant higher proportion of patients with acute rejection were induced with basiliximab (13 (92.8%) vs 1 (7.2%), p=0.001). The only thymoglobulin treated patient who experienced acute rejection had required dose reduction due to hematological complications. Patients with acute rejection had lower mean HLA mismatches (2.71 ± 0.83 vs 3.46 ± 1.41, p=0.011) and longer cold ischemia time, although not statistically significant (11.72 ± 5.39 vs 8.93 ± 3.56 hours, p=0.067) (Table 2).
Table 2. Clinical and analytical characteristics of recipients and donors according to biopsy results
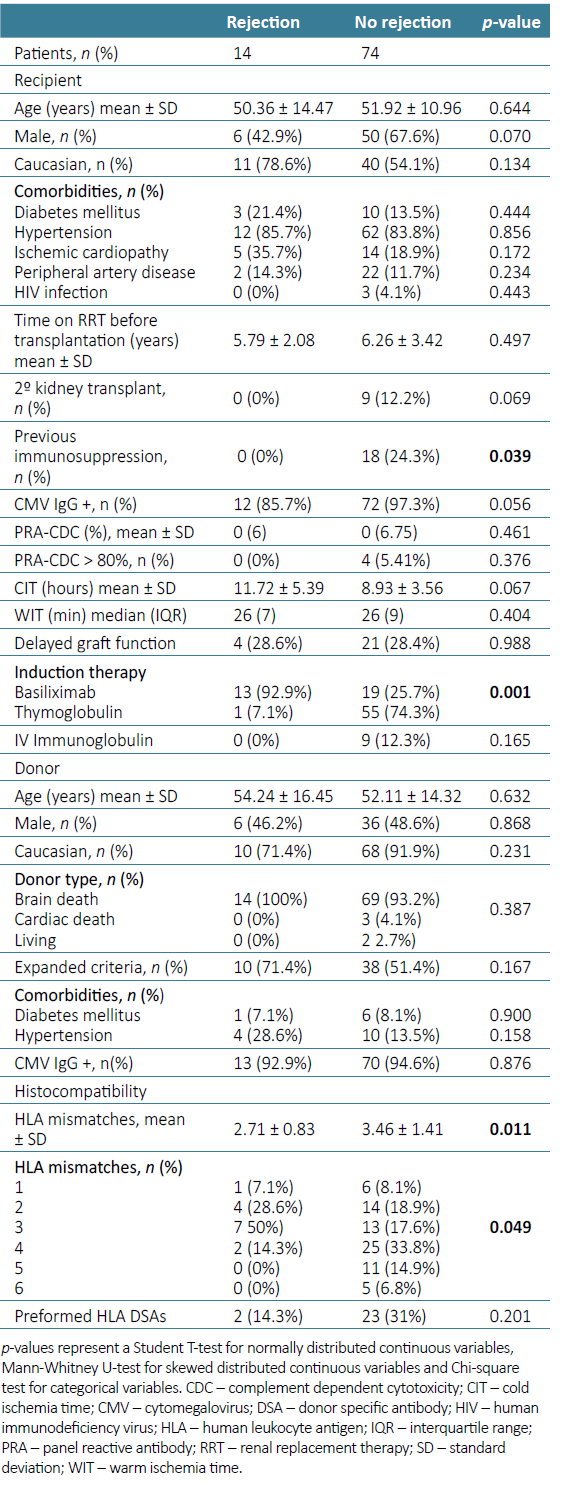
Table 3. Univariable and multivariable logistic regression analysis of predictors for acute rejection
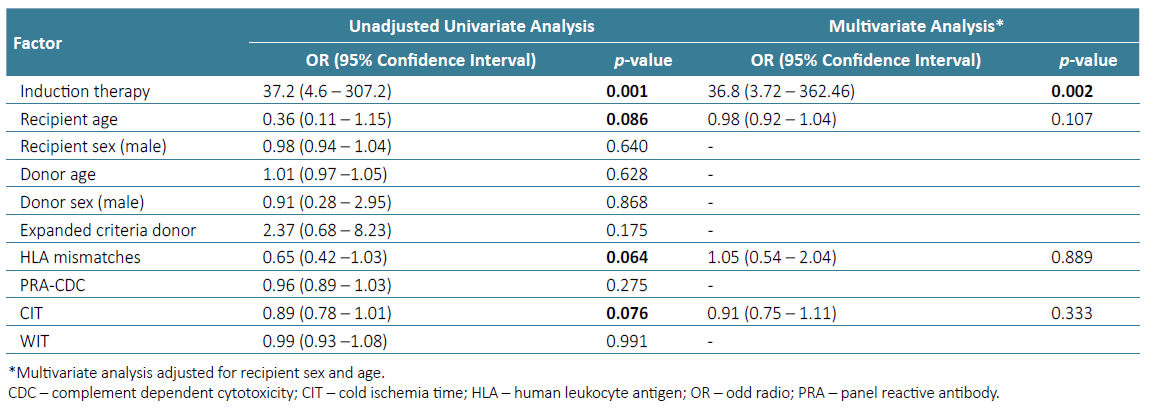
In a multivariate logistic regression analysis, adjusted for recipient age and sex, only induction therapy with basiliximab remained a strong predictor of early acute rejection [(OR) 36.8 (CI: 3.72 - 362.46), p=0.002].
A sub‑analysis that included only patients treated with basiliximab (n=32) was then performed and showed no statistically significant differences in clinical and analytical data, including low serum levels of TAC (<7 ng/mL) during the first week after transplantation, in those with and without acute TCMR or borderline rejection. All patients received 2 g of MMF daily.
DISCUSSION
Acute renal allograft rejection remains a major cause of allograft dysfunction. Even among patients who recover renal function, acute rejection is a major predictor of interstitial fibrosis and tubular atrophy which is responsible for most graft loss after the first year posttransplant.9 In the posttransplant period, acute rejection risk is largely determined by immunosuppression regimen and exposure. Currently, in the United States, 75% of kidney recipients receive thymoglobulin induction and > 90% maintenance immunosuppression consisting of TAC and MMF, as these regimens have been associated with lower rates of acute rejection.10 In fact, the advent of antiproliferative and calcineurin inhibitors lowered the incidence of acute rejection to less than 10% among the first year posttransplant.2 We report, however, a higher incidence of 15.9% of early TCMR and borderline rejection, which may be explained by the early setting of these protocol biopsies that mainly diagnose subclinical rejection. In fact, in our early rejection cohort, more than 70% of patients have subclinical rejection, with only 4 presenting delayed graft function requiring hemodialysis. Our results are similar to those previously described by Shapiro et al who reported borderline rejection in 21% and TCMR Banff I or II in 25% of protocol biopsies performed at a mean time of 8 days posttransplant,11 Moreso et al who described a prevalence of subclinical rejection of 14.2% in patients biopsied between 4 and 6 months post transplantation,12 and Rowshani et al who reported subclinical rejection in 15.2% in patients biopsied at 6 months.13 All patients received immunosuppression with TAC and MMF. Other studies, however, reported a much lower incidence of subclinical rejection, such as Rush et al, who presented an incidence of 4.6%, questioning the benefit of early protocol biopsies.14 Moreover, there is still uncertainty if inflammation in early protocol biopsy is related to alloimmune response versus response to ischemic injury, and most importantly whether it is associated with fibrosis progression and worst allograft outcomes. Mengel et al analyzed 833 protocol and 306 clinically indicated early biopsies and showed that the prevalence of inflammatory infiltrates was the same and that the presence of persistent inflammation on sequential biopsies was a significant determinant of allograft function, independent of an increased serum creatinine.15 Van Loon et al evaluated 329 biopsies performed in the first 14 days after kidney transplantation and confirmed that inflammatory lesions were often present. These changes were related to alloimmune risk factors, but not to nonimmune factors, such as ischemia times, older donor age or donor type, in their study. Graft dysfunction in the first 14 days translated into a worse graft survival beyond 14 days, independent of other baseline risk factors.16
In our cohort, most patients with acute rejection on early protocol biopsy were induced with basiliximab (13/92.8%), representing an incidence of 43.8% in the group (n=32) treated with this induction therapy. Interestingly, patients with acute rejection had lower mean HLA mismatches (2.71 ± 0.83 vs 3.46 ± 1.41, p=0.011), none had 5 or 6 HLA mismatches, none was receiving a second graft or was highly HLA sensitized (PRA >80%). Actually, these patients, classified as low ‑risk recipients, received per ‑protocol basiliximab induction therapy, the only factor that remained a strong predictor of early acute rejection in the multivariate logistic regression analysis. In patients with increased risk for acute rejection, including those with multiple HLA mismatches, PRA > 25%, preformed DSA, blood group incompatibility, CIT greater that 24 hours and younger recipient and older donor age, guidelines recommend induction therapy with thymoglobulin, since that regimen have been associated with lower acute rejection and graft loss rates.17 On the other hand, for low‑risk patients, the 2009 KDIGO clinical practice guidelines recommend the use of IL‑2 receptor antibodies as first‑line induction therapy, as data from randomized trials and retrospective studies have shown similar rates of acute rejection and patient and graft survival, with an increased risk of infectious and neoplastic complications in those treated with thymoglobulin.18,19Laftavi et al compared low‑dose thymoglobulin (3 ‑5 mg/kg total) and basiliximab in low‑risk patients defined as white, PRA < 30% and non‑donor with cardiac death recipients. In their study, low‑dose thymoglobulin was associated with lower rejection rate in living donor recipients and lower rejection and higher long‑term graft survival in deceased donor recipients, without differences in average hospital stay, malignancy rate or viral or bacterial infections.20 Martinez et al also reported similar rate of complications in low‑risk patients treated with low‑dose thymoglobulin or basiliximab,21 suggesting a potential role for this induction therapy in low‑risk renal transplant recipients. Our follow‑up period ended on the protocol biopsy date, so we collected no complications including infectious or neoplastic. Further studies comparing thymoglobulin and basiliximab induction therapy in patients defined as low‑risk, including efficiency and complications, will be needed to clarify our results better.
In our study, most patients with acute rejection diagnosed on protocol biopsy were Caucasian (11/78.6%), which contrasts with previous reports that described a higher rate of acute rejection in African‑American, even in those otherwise classified as low immunologic risk patients.22 In our transplant unit, patients with African ancestry primarily received thymoglobulin as induction therapy, which may have contributed to the lower rejection rate found in these patients in our cohort.
This study highlights the importance of protocol biopsies especially in patients with milder immunosuppressive regimens. However, the potential benefit must always be weighed against the risk of complications, including arteriovenous fistula, gross hematuria, or perinephric hematoma, occasionally requiring blood transfusion, radiological or surgical intervention, or rarely resulting in death. There were some limitations. First, this was a retrospective study with a small sample size. Second, this is not a randomized trial, and biases may exist. The absence of mid‑ and long‑term outcomes (e.g., kidney function or failure), prevents any definite conclusion about the efficacy of either induction regimen. Further studies should be performed to confirm our findings, comparing the efficacy and safety of basiliximab and thymoglobulin in low immunological risk patients, perhaps including thymoglobulin at lower doses.