INTRODUCTION
Peritoneal dialysis (PD) and hemodialysis (HD) are two very distinct forms of renal replacement therapy (RRT). Compared with HD, PD as an initial modality of RRT has been associated with better preservation of residual kidney function (RKF) and improved survival, especially during the first two years of treatment.1‑3
However, a significant proportion of PD patients begin RRT through HD before transitioning to PD, and the reasons found in the literature are diverse. One of the most important causes is late referral to nephrology, which translates in to limited or no exposure to pre‑dialysis care and renal replacement therapy education ‑ this group includes chronic kidney disease patients who received no prior nephrology care and patients with acute kidney injury (or acute kidney injury superimposed on chronic kidney disease) requiring urgent dialysis initiation. On the other hand, in some cases, despite receiving proper follow‑up and education, delays related to catheter placement may lead to an initial period of HD before PD initiation. For some patients, a change of preference, social situation, living conditions, or health status may also result in a transition to PD.4,5
Some studies have suggested that an initial period on HD may be associated with adverse outcomes once patients transition to PD.4‑8Possible mechanisms might include a more rapid loss of RKF during the initial HD period, a greater difficulty to adjust to the autonomy of PD after being exposed to in‑center HD, and more severe illness in the group of patients requiring an initial period of HD.4 A potential solution to avoid having to go through the HD initial period, besides the long‑term advocated early referral to nephrology, is the implementation of urgent PD start programs, since it has been shown that it can be as feasible, safe and with good outcomes as a programmed‑start PD.9,10
Given that, the primary objective of our study was to evaluate the impact of an initial period on HD before transitioning to PD on clinical, analytical and treatment related outcomes, one and two years after the beginning of RRT, since studies directly comparing these two groups of patients are lacking.
MATERIAL AND METHODS
We conducted a retrospective cohort study, which included the prevalent patients in our PD unit between January 1st 2017 and December 31st 2019. Patients were included if they were older than 18 years and had at least one year of PD vintage. We excluded patients submitted to kidney transplant with a failing graft. We reviewed patient’s medical record and collected demographic and clinical information at the time of PD start as well as one and two years after. Baseline data included age, gender, end‑stage renal disease (ESRD) etiology, presence of diabetes, hypertension, dyslipidemia, obesity (body mass index > 30 kg/m2) or heart failure, need for an initial period on HD and the time and associated reasons. We also collected data including residual glomerular filtration rate (GFR), residual diuresis, weekly Kt/V and number of antihypertensive drugs needed for blood pressure control. We also registered the number of cardiovascular events (acute myocardial infarction, cerebrovascular accident), peritonitis and the PD drop‑out, its motives, and mortality on the first two years. Residual GFR was calculated as the average of 24‑hour urinary creatinine and urea clearances. When data was missing, we estimated GFR using modification of diet in renal disease equation.
The patients were divided in two groups, one including the patients who initiated RRT with PD (“PD‑first” group), and other with the patients who initiated RRT with intermittent HD and who switched to PD within the first six months of RRT initiation (“HD‑first” group); patients with longer HD time were excluded. All patients on the “HD‑first” group were submitted to a standard hemodialysis prescription, with 3 or more sessions per week of at least 3 hours.
Statistical analysis was performed using IBM‑SPSS Statis‑ tics v22 and the confidence interval was set on 95%. A p value < 0.05 was considered statistically significant. Mean values and standard deviations were calculated. Comparison of means and frequencies of normally distributed variables were calculated using t‑tests and the χ2 test. Pearson’s correlation was used to identify a correlation between different variables. Independent samples t‑test was used to compare demographic data, prior diseases, PD‑related parameters.
RESULTS
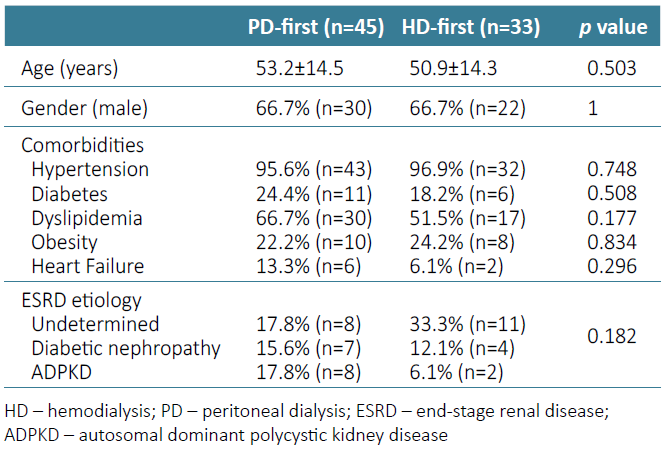
We included 78 PD patients in our study, mostly Caucasian (97.4%; n=76), male (66.7%; n=52), with a mean age of 52.2±14.3 years. Twenty‑four percent (n=19) of the patients had ESRD of undetermined cause, and the most prevalent identified cause of ESRD was diabetic nephropathy (14.1%; n=11) followed by autosomal dominant polycystic kidney disease (ADPKD) (12.8%; n=10). Regarding the comorbidities, we verified a very high prevalence of high blood pressure (96.2%; n=75) and dyslipidemia (60.3%, n=47), followed by diabetes (21.8%, n=17), obesity (23.1%, n=18) and heart failure (10.3%, n=8). There were no statistically significant differences between the two groups (HD‑first and PD‑first) concerning age, gender, prevalence of comorbidities or ESRD etiology (Table 1).
Table 1. Characteristics of PD‑first and HD‑first groups regarding demographic data and baseline comorbidities
From the 78 patients, 33 (42.3%) initiated RRT with intermittent HD and switched to PD after a median time of 3 months (interquartile range (IQR 2‑4.5)). The main reason found to go through a HD period was the need for urgent start of RRT (84.8%; n=28), in the setting of an acute kidney injury in patients with chronic kidney disease (CKD) due to an acute event, such as infection or cardiovascular event. Other causes for HD to PD transition were: change of patient’s option after HD start (9.1%, n=3) and PD catheter’s malfunction at the moment of PD start that could not wait for the surgical revision (6.1%, n=2). Most of this sub‑population had previous follow‑up by a nephrologist (82.1%, n=23) and had received specific education on the different RRT available (69.6%, n=16) and had chosen PD, so they started HD using a central venous catheter. All PD‑first patients started in a programmed setting and no urgent PD starts were registered.
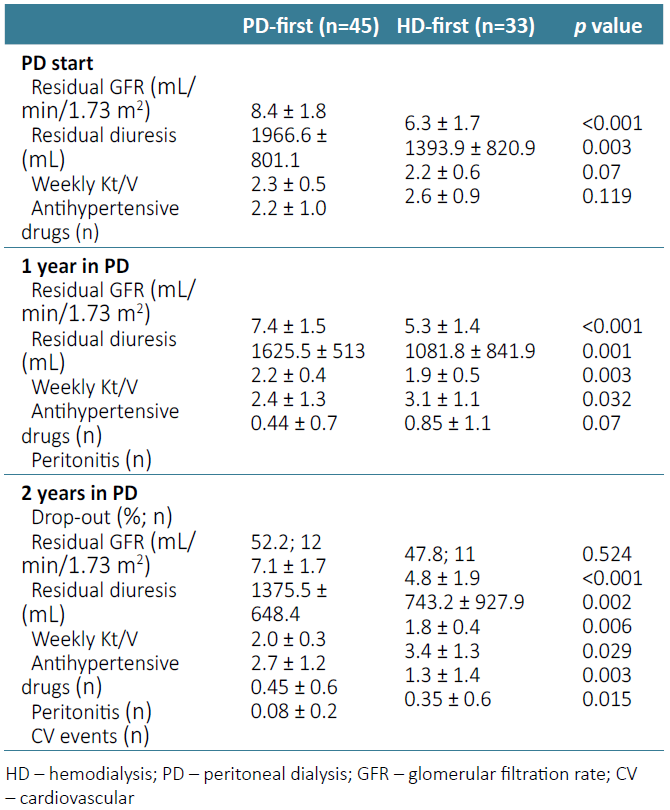
At moment of PD start, when comparing the two groups, we found that patients who went through a period of time in HD had a significant lower volume of residual diuresis (1393.9 ± 820.9 mL vs 1966.6 ± 801.1 mL; p=0.003) and a significant lower residual GFR (6.3 ± 1.7 mL/min/1.73 m2 vs 8.4 ± 1.8 mL/min/1.73 m2; p<0.001), with no statistically significant difference concerning weekly Kt/V (p=0.07) (Table 2).
One year after stating PD, the “HD‑first” patients , needed a higher number of antihypertensive drugs to maintain a normal blood pressure (3.1 ± 1.1 vs 2.4 ± 1.3; p=0.032), had a lower volume of residual diuresis (1081.8 ± 841.9 mL vs 1625.5 ± 513 mL; p<0.001) associated with lower residual GFR (5.3 ± 1.4 mL/min/1.73 m2 vs 7.4 ± 1.5 mL/min/1.73 m2; p<0.001), and, consequently with lower weekly Kt/V (1.9 ± 0.5 vs 2.2 ± 0.4; p=0.003) as the PD weekly Kt/V is based in the kidney residual function. The total number of episodes of peritonitis during the first year was 28 (0.36 episodes/patient/year) and was higher in the patients that started with HD (0.85 ± 1.1 vs 0.44 ± 0.7 episodes/year; p=0.07), however it did not reach statistical significance. Despite this result, after performing a logistic regression analysis, we found that the occurrence of peritonitis did not prove to be related to lower residual diuresis, GFR or dialysis efficacy in this group of patients (Table 2).
Two years after PD initiation 55 of the initial 78 initial patients remained (70.5%) on PD. In these patients we found that the tendency described before was similar at the end of the follow‑up. Patients from the HD‑first group needed a higher number of classes of blood pressure control drugs (3.4 ± 1.3 vs 2.7 ± 1.2; p=0.029), had inferior residual diuresis volume (743.2 ± 927.9 mL vs 1375.5 ± 648.4 mL; p=0.002), GFR (4.8 ± 1.9 mL/min/1.73 m2 vs 7.1 ± 1.7 mL/min/1.73 m2; p<0.001) and weekly Kt/V (1.8 ± 0.4 vs 2.0 ± 0.3; p=0.006). The number of episodes of peritonitis (n=21, 0.38 episodes/patient/year) was also higher in this group of patients (1.3 ± 1.4 vs 0.45 ± 0.6 episodes/year; p=0.003), and it was once again unrelated with lower residual diuresis (p=0.2), GFR (p=0.7) or dialysis efficacy (p=0.09). At the end of the follow‑up, it was also noticed that the patients in the “HD‑first” group also had significantly more cardiovascular events (0.35 ± 0.6 vs 0.08 ± 0.2 events; p=0.015) (Table 2).
Considering the 23 patients that did not reach the end of the follow‑up, 11 (47.8%) were from the “HD‑first” group and 12 (52.2%) were from the “PD‑first” group. The main reason for PD dropout was kidney transplant (65.2%; n=15), followed by ultrafiltration failure (26.1%; n=6), refractory peritonitis (4.3%; n=1) and death (4.3%; n=1). Regarding the patients that had ultrafiltration failure, four (66.6%) were from the “HD‑first” group and it happened 17.3 ± 6.3 months after PD start. Only one patient died during follow‑up from an acute cardiovascular event.
DISCUSSION
In our series we found that patients who go through an initial period on HD have lower volume of residual diuresis and of residual GFR when starting PD and during the follow‑up. Additionally, they also have the need for a higher number of different antihypertensive drugs to maintain a normal blood pressure, lower weekly Kt/V and higher number of peritonitis and cardiovascular events.
In our study group 42.3% of the patients initiated RRT with intermittent HD and switched to PD, mainly due to urgent need of RRT. As showed in our results, and reiterated in other studies, this HD period might have significant long‑term consequences.4 Therefore, the development of programs that include the possibility of urgent PD start might help reduce this number and are an important strategy to promote home dialysis,9‑12with fewer or at least similar short‑term and intermediate‑term complications compared with urgent‑start HD.11,13However, difficulties often arise to implement such programs, as we experience in our center, due to the need of a specialized team to always be on hand to insert the peritoneal catheter and a trained and specialized team for education and technique support throughout the initiation of the modality.9 Regarding the main differences found between the two groups of patients, it is likely that the lower volume of residual diuresis, residual GFR, lower weekly Kt/V and the higher need for different antihypertensive drugs to maintain a normal blood pressure are related to each other and are due to the hemodynamic flux variations that occur during their time on HD that cause a quicker loss of the RKF.4,14,15Furthermore, better preservation of RKF has been associated with better clinical outcomes for both HD and PD patients justified by the small‑solute clearance as well as importance in several metabolic, humoral, and hemodynamic functions.14 Therefore, when there is no alternative to HD start and in patients who are known to want to transition to PD, it is important avoid excessive ultrafiltration rates and intradialytic hypotension to reduce the loss of RKF and diuresis16 and, also, adopting an incremental personalized HD prescription may also be beneficial.17
The “HD‑first” group also experienced higher rates of peritonitis in second year of follow‑up, which is not surprising since transfer from HD to PD has been shown to be an independent risk factor for the development of peritonitis on several studies.4,15,18This fact may be attributed to the greater difficulty of adjusting to a self‑dialysis technique as PD, comparing to an assisted‑dialysis technique, as in‑center HD, or to the fact that loss of RKF has shown to be a possible predictor of peritonitis in some studies.4,18Regarding the six patients who had ultrafiltration failure as the cause for PD dropout, the majority were from the “HD‑first” group, which meets the results from different studies that show that patients who transferred from HD to PD have a 21% - 33% increased risk of PD technique failure relative to incident PD patients.4,10,19Despite these differences, it is important to note that, even though they have lower average weekly Kt/V, it is possible to achieve good dialysis adequacy in the “HD‑first” group, as shown by our results. The higher incidence of cardiovascular events in the patients previously submitted to a HD period has also been described in other studies and it is possible that HD could induce sub‑clinical cardiac ischemia, left ventricular hypertrophy and promote an inflammatory state which might increase the risk of subsequent cardiovascular events even once a patient has transitioned to PD.4,21,22However, several studies have contradicting data on this subject and the risk for cardiovascular events is probably a combination of the patient’s comorbidities and inadvertent effects of RRT, as both techniques are associated with increased risk of cardiovascular events.23 Finally, a difference in mortality or rate of technique failure between the two groups was not observed in our patients, probably due to the follow‑up time of two years.
Our study presents some limitations, on the one hand we present a relatively small sample compared with other studies and with a follow‑up of only two years. On the other hand, when comparing the two groups there are differences that cannot be ignored, such as the conditions of RRT start, with more urgent and unstable patients on the “HD‑first” group, or other patient´s characteristics that could influence dialysis adequacy or risk of peritonitis that were not accounted for, such as peritoneal membrane characteristics, the PD solution used and performance of invasive procedures. However, the two groups present similar baseline features which allows for an unbiased general comparison.
In conclusion, a period of time in HD prior to PD, probably related with periods of higher hemodynamic instability, seems to precipitate a faster reduction of RKF and, consequently, of residual diuresis and dialysis efficacy. In these patients we also identified a higher risk of peritonitis and cardiovascular events. With our findings we reinforce the importance of promoting the start of PD‑first, especially if this was already the patient’s option in the process of the education of CKD and RRT available. Also, efforts should be made to create the adequate infrastructure and a trained staff to offer a program of urgent‑start PD, since it has been shown that it can be as feasible, safe and associated with good outcomes. Moreover, when a period of time in hemodialysis is inevitable, a personalized incremental HD prescription should be considered, as it may possibly change the long‑term outcomes. Furthermore, it is important to increase vigilance among the switched patients concerning training, peritonitis prevention practices, adequate psycho‑social support, and monitoring for RKF loss.