INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease that is associated with significant organ damage, morbidity and mortality. Approximately 15%-20% of SLE patients are diagnosed during childhood, with dis‑ ease onset prior to 18 years of age.1 It is a relatively rare pediatric disease with an annual incidence of 0.3-2 cases per 100 000 childhood population, with variation according to ethnicity. In fact, the disease is more prevalent in patients of black or Asian descent.2 Median age of onset is between 11 and 12 years and 80% of patients are female.3
Childhood‑onset SLE is recognized to have a more active disease course when compared with those presenting in adulthood, with lupus nephritis (LN) being more frequent.4 LN is defined as histologically proven disease and occurs in around 50%-82% of children in comparison with 20%-40% of adults.2 The majority of histologically proven disease is class IV LN, the most active disease class associated with the worst kidney prognosis.5
Patients may present with asymptomatic hematuria, mild proteinuria, nephrotic syndrome, rapidly progressive glomerulonephritis, acute or chronic kidney injury.3,6Early diagnosis and treatment are important as LN can lead to the development of chronic kidney disease (CKD), end‑stage kidney disease (ESKD), and mortality.
Despite the availability of effective immunosuppressive therapies, treatment response remains suboptimal, with 50% to 78% patients in full remission at 24 months based on various criteria for response.7 The Kidney Disease Improving Global Outcomes (KDIGO) guidelines8 suggest similar induction and maintenance therapy regimes to the ones used in adults, however, advise to consider issues relevant in this population, such as dose adjustment, growth, fertility, osteonecrosis of femoral head, and psychosocial factors, when choosing the therapy plan.
Transition to adulthood is crucial in patients with LN with childhood onset, as they have an increased risk of progression to ESKD especially those who do not achieve complete remission. They also often require increased dosages of corticosteroids as well as an overall increased number of immunosuppressive medications to achieve remission.1,7Therefore, the aim of our study was to evaluate clinical presentation of LN with childhood‑onset, the treatment regimen instituted and the transition to adulthood.
SUBJECTS AND METHODS
We conducted an observational retrospective study including patients diagnosed with LN during childhood that were followed by pediatric nephrology with subsequent transition to adult nephrology in the period between 1995 and 2020.
Demographic data, such as gender, ethnicity and age (at the time of diagnosis and current), and also, clinical data, as presentation and clinical development of the disease, classification of the kidney biopsy and induction therapy, were collected retrospectively from the existing patient records. Variables such as maintenance therapy, estimated glomerular filtration rate (eGFR), proteinuria, complement (C3, C4), autoimmunity (antinuclear antibodies, anti‑double‑stranded DNA), number of flares and treatment, were collected at the diagnoses, time of transition to adulthood and currently.
According to KDIGO guidelines, proteinuria was quantified based on a protein‑creatinine ratio from an isolated sample of urine. Normal values were considered <0.2 mg/mg or <20 mg/mmol in children >2 years old and <0.5 mg/ mg or <50 mg/mmol in children between 6 months and 2 years. Mild proteinuria was considered between 0.2‑2 mg/mg or 20‑200 mg/mmol. Nephrotic range proteinuria was considered >2 mg/mg or >200 mg/mmol. Response to treatment was also registered using the values of protein‑creatinine ratio (PCR) and eGFR. Complete response (CR) was defined as reduction in proteinuria <0.5 g/g (50 mg/ mmol) and stabilization or improvement in kidney function (± 10%‑15% of baseline) within 6‑12 months of starting therapy, but could take more than 12 months. Partial remission (PR) was defined by a reduction in proteinuria by at least 50% and to <3 g/g (300 mg/mmol) and stabilization or improvement in kidney function (± 10% ‑ 15% of baseline) within 6‑12 months of starting therapy. For children < 18 years old, complete response is defined as proteinuria <0.5 g/1.73 m2/d or < 300 mg/m2/d.8 Patients who did not achieve CR or PR were defined as non‑responders. Progression to ESKD, defined as eGFR lower than 15 mL/min/1.73 m2 or need of kidney replacement therapy, or death were also registered.8,9
Estimated GFR was calculated during childhood using the revised Schwartz equation and in adulthood using the Chronic Kidney Disease Epidemiology Collaboration (CKD‑EPI) 2021 equation.
The statistical analysis was performed using IBM SPSS Statistics version 28 software (IBM, Armonk, NY, USA). A descriptive analysis was conducted, nominal variables were described using frequency tables, and continuous variables were described using mean values and standard deviations. Comparison of frequencies was made between variables of interest. A p‑value < 0.05 was considered statistically significant.
Clinical and laboratory characteristics
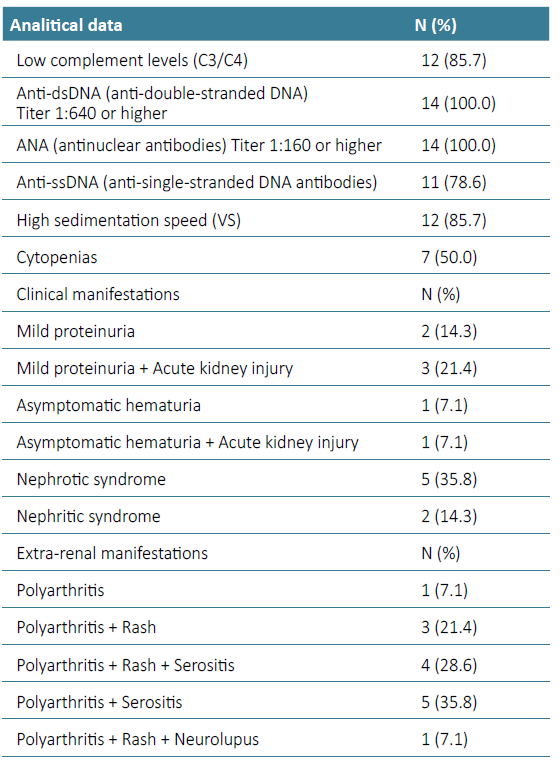
Of the 14 patients with LN included in this study, 3 were males (21,4%) and 11 were females (78.6%), with a mean age of 26.3 ± 5.2 years (minimum, 21 years; maximum, 44 years). The mean age at diagnosis was 13.6 ± 2.49 years (minimum, 9 years; maximum, 17 years). The main clinical manifestations and laboratory indicators at diagnosis are shown in Table 1. The most common presentation was proteinuria (71.4%). Half of these patients (50.0%) had mild proteinuria ‑ median 1.13 g/day (Inter quartile range (IQR) 0.33-2.41 g/day) ‑ and the other half had nephrotic‑range proteinuria ‑ median 3.77 g/day (IQR 3.43-4.51 g/day). Acute kidney injury was observed in 4 (28.6%) children at diagnosis. Of initial non‑renal manifestations, polyarthritis (100.0%) and rash (57.1%) were the most common. Females presented more often with rash than males (n=7 vs n=1, p=0.026). One patient (7.1%) had neurolupus as initial manifestation. At presentation, the patient with neurolupus had an extensive hemorrhagic cerebral infarction with subsequent neurological sequelae. Antinuclear antibodies (ANA) and anti‑double‑stranded DNA (anti‑dsDNA) antibodies were detected in all patients (100.0%), followed by anti‑single‑stranded DNA (anti‑ssDNA) antibodies (78.6%).
Pathological characteristics
All children willingly underwent kidney biopsy. Class IV LN was the most common (11 cases, 78.6%), there were 2 cases (14.3%) of class V LN and one case (7.1%) of class III LN. No class VI LN was found among the children in this study. We compared the main laboratory indices at diagnosis of children with class III LN, class IV LN and class V LN. We found significant differences in quantitative levels of 24‑h proteinuria and eGFRs among the three groups of children. In fact, quantitative levels of 24‑h proteinuria were significantly higher in children with class V LN than in those with class III LN (median 24‑h proteinuria 3.71 vs 1.45 g/day, p=0.017) and class IV LN (median 24‑h proteinuria 3.71 versus 2.55 g/day, p=0.029).
Induction and maintenance therapy
In initial therapy, all children were treated with an induction regimen of corticosteroids combined with other immunosuppressants, primarily cyclophosphamide (CYC) (n=5, 35.3%), or mycophenolate (MMF) (n=9, 64.7%). In maintenance therapy, all children were treated with a regimen of corticosteroids combined with primarily CYC (n=6, 42.9%) with posterior transition to MMF, or MMF (n=5, 35,7%) or azathioprine (n=3, 21.4%).
Follow‑up, prognosis and renal outcomes
The median follow‑up duration was 112.2 months, with the shortest 44.2 months and the longest 242.6 months. A complete remission occurred in all patients.
A total of 6 patients (42.9%) had renal flares equally distributed in youth or adulthood. All these patients under‑ went kidney biopsy and we had 2 cases (33.3%) of class III LN, 2 cases (33.3%) of class IV LN and 2 cases (33.3%) of class V LN. All treated with intravenous cyclophosphamide with complete remission in 83.3% of patients (n=5) and partial remission in the other 16.7% of patients (n=1). Looking back at induction therapy of these patients, they underwent MMF in 50.0% of cases (n=3) and CYC in the other 50.0% of cases (n=3). At the time of the renal flare, all the six patients were under treatment with MMF.
In the transition to adulthood, MMF was discontinued in 3 patients (21.4%) after 17±9 months. At the end of follow‑up, 11 patients (78.6%) are with MMF and oral corticosteroids and 3 patients are with oral corticosteroids. The daily dose of glucocorticoids was inferior to 7.5 mg/day of prednisone in all patients.
None of these patients developed end‑stage kidney disease or died. At the end of follow‑up, arterial hypertension was present in 28.6% (n=4), proteinuria >0.5 g/day was present in 35.7% (n=5) (IQR) 0.55-0.71 g/day) and chronic kidney disease stage 3‑4 was present in 42.8% (n=6). Of the five patients with proteinuria >0.5 g/day, four have arterial hypertension (80.0%) and three have diabetes (60.0%). We report three patients with chronic kidney disease stage 3a (50.0%), two patients with chronic kidney disease stage 3b (33.3%) and one patient with chronic kidney disease stage 4 (16.7%).
DISCUSSION
SLE is a chronic autoimmune disease, and approximately 20% of cases presents in childhood.1 The disease manifestations of pediatric SLE are often more serious, especially for multiple organ functions such as those of the renal, nervous, and circulatory systems.2 As one of the most common complications of SLE, LN is also one of the most common causes of secondary glomerulonephritis affecting the long‑term prognosis of children. LN has various clinical manifestations and pathological types in children, varying from mild hematuria or proteinuria to nephrotic syndrome and kidney failure. The high disease activity of SLE and the toxicity related to long‑term use of corticosteroids and immunosuppressive agents cause serious damage to the body.3
In our study, the epidemiological characteristics and clinical manifestations were similar to previous reports. The initial manifestation of kidney involvement was mainly proteinuria. Of initial non‑renal manifestations, polyarthritis and rash were the most common. Compared with adult patients, symptoms such as oral ulcers and alopecia in pediatric patients were relatively rare10 and we have none to describe.
Only one child with LN in our center had neuropsychiatric symptoms. However, literature shows that nervous‑system involvement is an independent risk factor affecting prognosis in pediatric SLE, and is also an important cause of death (cerebral hemorrhage and cerebral hernia).10,11Children with nervous‑system involvement are sometimes underrecognized in the early stage due to mild or asymptomatic clinical presentation, leading them to be misdiagnosed or undiagnosed. However, once severe symptoms such as disturbance of consciousness, epilepsy, and brain herniation occur, the disability and fatality rates of these patients are relatively high. Therefore, it is necessary to be vigilant in clinical practice. Electroencephalography, cerebrospinal fluid tests, head imaging, and other auxiliary examinations should be used, when considered appropriate and not always, to comprehensively evaluate children with SLE.10
Kidney biopsy data in this study showed that class IV LN was the most common pathological type. These findings were consistent with those of another studies.10‑12Urine protein level was significantly higher in class V LN and kidney function was better in class III and IV LN than in class V LN. In the course of disease progression, the pathological type of LN can also change. Repeat kidney biopsy is also recommended in some cases, such as worsening or refractoriness to treatment.10 In this study, the data showed that six patients underwent kidney biopsy again. So, when children with SLE have evidence of kidney damage, a timely kidney biopsy is recommended to clarify the pathological type and provide reasonable treatment in time.10
It is imperative to underscore that the occurrence of proteinuria in five patients at the conclusion of the follow‑up period is intricately linked to the protracted course of kidney disease, compounded by the presence of concurrent comorbidities such as hypertension and diabetes.
Compared with adults, children have more active forms of SLE and receive more intensive immunosuppressive therapy.13,14In fact, this means a long‑term use of steroids and immunosuppressants which are toxic and accumulate more organ damage over time. Several issues must be addressed when treating pediatric lupus, including adherence concerns, which may favor intravenous medications; growth concerns, which may favor limiting glucocorticoid exposure; fertility concerns, especially as patients approach adolescence, which may favor limiting cyclophosphamide exposure; and psychosocial concerns relating to school and socialization with peers.8
Given that there are few children included in clinical trials for LN, the treatment of LN in pediatric SLE is largely extrapolated from adult data. Treatment of pediatric LN may include high‑risk immunosuppression with CYC. Risk of infection and concern about cumulative toxicity are significant considerations when initiating treatment with CYC.15 In our study we have an elevated use of CYC as induction therapy. In fact, there are several studies comparing CYC with MMF that describe that the efficacy of MMF is non‑inferior to CYC for pediatric LN treatment.16 The high use of CYC in our study is related to the fact that the sample includes patients since 1995, with the start of use of MMF after that date. As well as the intolerance of some patients to the MMF induction dose, particularly due to gastrointestinal adverse effects.
Emerging therapeutic antibodies have become an alternative for the treatment of SLE, such as rituximab and belimumab.17 Rituximab is an established therapy in children with idiopathic nephrotic syndrome to sustain short‑ to medium‑term disease remission and avoid steroid toxicities.18 According to the literature, rituximab is an effective and safe rescue therapy for childhood‑onset LN patients with life‑/organ‑threatening manifestations or treatment‑resistance.19
Belimumab is a recombinant, immunoglobulin G1λ human monoclonal antibody that antagonizes biological activity of soluble BAFF.20 Based on clinical‑trials, belimumab is useful and safe in adult SLE and LN. The evidence of belimumab effectiveness in pediatric SLE and pediatric LN is limited.21 Although, there are studies that confirm that belimumab intravenous pharmacokinetics and benefit-risk profile in childhood SLE are consistent with adult belimumab studies. 20
There are currently some clinical trials being carried out in order to evaluate the applicability of new drugs in a pediatric population with LN, namely obinutuzumab and voclosporin.22
In conclusion, the long‑term outcome in this group of children with LN was excellent, with 100% patient and renal survival. Nevertheless, is important to notice the high dependence of immunosuppressive agents in adulthood probably related with severe disease at childhood‑onset. The diagnosis of LN in pediatric age has its particularities and constitutes a challenge for the nephrology community. Biological therapy currently shows promise in improving the prognosis of these patients.