INTRODUCTION
Secondary hyperparathyroidism (SHPT) is a disorder in patients with chronic kidney disease (CKD), characterized by excessive production and secretion of parathyroid hormone (PTH) and parathyroid hyperplasia as a consequence of vitamin D deficiency and calcium and phosphorus renal metabolism imbalances (hypocalcemia and hyperphosphatemia).1,2High PTH levels usually occur as early as CKD stage G2,3 affecting 40% of stage G3 CKD patients and 80% of stage G4 CKD patients.4,5Without effective control, SHPT becomes progressively more severe and refractory to medical treatment, which can lead to parathyroidectomy.6 The clinical recommendations for treatment are available for dialysis stage G5 CKD patients, but robust data focusing on PTH optimal levels and therapeutic strategies to support clinical decisions regarding pre‑dialysis stage G3‑G4 CKD patients is still missing.7 The Kidney Disease: Improving Global Outcomes (KDIGO) 2017 guidelines8,9recommend regular monitoring of PTH levels starting in stage G3a CKD to identify patients with progressively rising or persistently elevated PTH levels above the standard upper limit (usually 65 pg/mL). Thus, patients at risk can be identified and evaluated for modifiable risk factors or surrogate markers such as calcium, phosphorus, vitamin D and PTH levels to assess the disease progression.8 Hyperphosphatemia does not appear early, as phosphate renal fractional excretion of phosphate increases in CKD patients. This is mainly due to the increase in fibroblast growth factor‑23 (FGF‑23), which increases renal phosphate excretion but decreases serum concentration of calcitriol/1.25(OH)2D3 - well‑known drivers of vitamin D deficiency.10 Vitamin D deficiency (defined as serum calcidiol/25(OH) D <30 ng/mL)11 is related to increased PTH levels in non‑dialysis CKD patients,12,13affecting 71% and 84% of stage G3 and G4 CKD patients, respectively.14
The KDIGO guidelines recommend that stage G3‑G4 CKD patients with progressively rising or persistently elevated PTH levels above 2 to 9 times the standard upper limit should be evaluated for vitamin D deficiency.8 Supplementation with native vitamin D (ergocalciferol, cholecalciferol) to increase the rate of bioactivation into active vitamin D (calcitriol) in G3a‑G3b CKD patients, has been under investigation for many years, despite its modest efficacy in lowering PTH levels and the lack of a consensual appropriate target for serum total 25(OH)D and optimal dosing regimen.15 New data has also demonstrated that obesity constrains the effectiveness of cholecalciferol and ergocalciferol, due to the accumulation in adipose tissue of these nonpolar molecules, where they become largely unavailable for hepatic conversion to 25OHD.16 Thus, as CKD patients tend to be overweight or obese, the clinical benefit of native vitamin D to treat SHPT becomes limited.16‑18
Bioactive vitamin D (calcitriol) and active analogues (paricalcitol and alfacalcidol) have demonstrated therapeutical potential for SHPT in non‑dialysis CKD patients by suppressing PTH secretion.14 Still, these are associated with an increased risk of hypercalcemia and exacerbation of hyperphosphatemia.19 Hence, the 2017 KDIGO guidelines’ update removed the recommendation for routine use of calcitriol or active analogues in G3a‑G5 CKD, reserving their use for patients with G4‑G5 CKD with severe and progressive SHPT.8 An extended‑release calcifediol (ERC) is a new calcitriol prohormone formulated for a slow and steady release over an extended 12 hours‑period, overcoming the catabolic feedback loop that degrades active vitamin D in inactive forms, suppressing PTH synthesis and secretion.20 The efficacy and safety of oral ERC in patients with G3‑G4 CKD were already demonstrated in phase 3 clinical trials.21‑23ERC treatment (30 or 60 μg/day dosage) significantly increased mean serum total calcidiol/25(OH)D levels to ≥50 ng/mL, with a concomitant decrease of PTH serum levels by >30%, with no significant impact on calcium, phosphate, or FGF‑23 levels.24 This agrees with the requirement of 25(OH)D levels ≥50 ng/mL for effective control of SHPT in pre‑dialysis CKD patients.24,25
Despite promising, clinical studies with ERC did not fully demonstrate the halt of SHPT clinical disease burden, hence why ERC is still not recommended by KDIGO.8 To further assess the efficacy of ERC, two clinical trials are currently ongoing on SHPT patients: NCT05460234 (PORTRAY study; observational study in non‑dialysis CKD patients; last patient recruited on January 2024) and NCT03602261 (in CKD patients requiring regular hemodialysis; placebo‑controlled phase 2 study with active status but not recruiting, at the time of manuscript preparation). Regimens for SHPT management can also include PTH suppression through calcimimetics (e.g., cinacalcet),26 albeit increased risk of (asymptomatic or mild) hypocalcemia - due to this, its usage is limited for the chronic dialysis population.8,27Concomitant therapy to lower serum phosphorus concentrations is also frequently prescribed through dietary phosphate restriction and administration of phosphate binders (e.g., calcium carbonate, calcium acetate, sevelamer carbonate, and others).28 Although effective, some of these agents have an associated risk of hypercalcemia (calcium‑based binders), which can be surpassed with equally or slightly less effective calcium‑free binders.29 Despite some progress, the optimal management of SHPT in non‑dialysis G3‑G4 CKD patients remains challenging and not consensual, as addressed by KDIGO,8 thus hindering the establishment of therapeutic guidelines and standards of care in Europe. Moreover, new therapeutic approaches with apparent positive effects on biochemical and clinical endpoints of SHPT in pre‑dialysis CKD are urgently needed to draw guidelines among the experts. Thus, this study discloses the results of a Delphi‑like questionnaire that assessed the knowledge and practice in diagnosing and treating SHPT in CKD patients (G3‑G4) among Portuguese nephrologists.
METHODS
This cross‑sectional survey study assessed the Portuguese nephrologists’ agreement level regarding the diagnosis and treatment of SHPT in pre‑dialysis CKD patients (stage G3‑G4). For that, 17 statements were defined and validated by a group of experts (focus group) in SHPT in pre‑dialysis CKD, through iterative rounds of discussion (Fig. 1; Table 1). The final questionnaire of 17 statements was uploaded to an online platform to execute a one round Delphi‑like panel (DP) that was responded to by 59 nephrologists from a universe of 550 nephrologists from the Portuguese Society of Nephrology (SPN). The questionnaire link was disseminated to the participants through SPN internal mailing list.
For each statement, the participants answered (one‑single response per statement) using a 4‑point Likert scale (“fully agree”, “agree”, “disagree”, “fully disagree”), and the answers were kept anonymous and confidential. The consensus agreement level was set at 70% of responses “fully disagree” or “fully agree”. Combined levels of at least 70% in terms of agreement (i.e., “agree” and “fully agree”) or disagreement (i.e., “disagree” and “fully disagree”) were categorized as a qualified majority.

Figure 1. Methodology of the single‑round Delphi‑like panel. CKD: chronic kidney disease; DP: Delphi‑like panel; SHPT: secondary hyperparathyroidism.
RESULTS
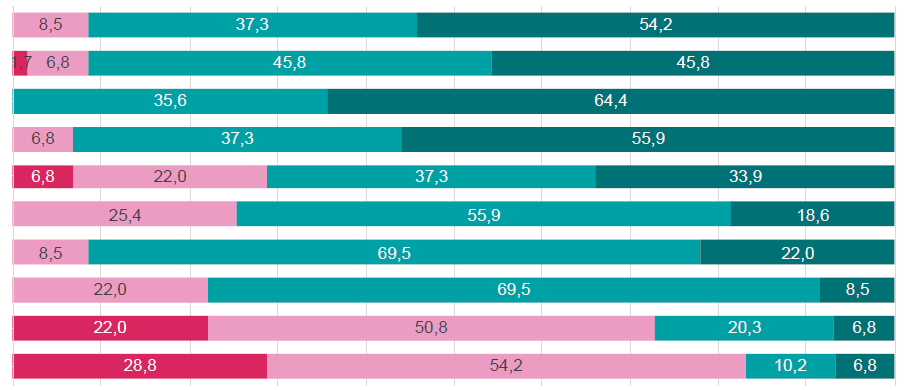
In this one‑round Delphi‑like panel, no statements were categorized as consensus, and 10 out of 17 statements (58.8%) reached a qualified majority of agreement/dis‑ agreement (Fig. 2 and Table 1).
The majority of the participants agreed that:
SHPT is a frequent and essential complication of CKD stage G4 adult patients.
Serum calcium and phosphate levels should be monitored starting at CKD stage G3a, at intervals of every 6‑12 months, and monitoring of PTH should be based on baseline level and CKD progression.
Stage G4 CKD patients should have calcium and phosphate monitored every 3‑6 months and PTH every 6‑12 months.
Stage G3a‑G4 CKD patients’ treatment through a regimen of native vitamin D to correct vitamin D insufficiency or deficiency, followed by administration of active vitamin D or analogues to manage SHPT.
Precisely, native vitamin D supplementation is estimated to be administered to <70% of CKD G3a‑G4 patients.
Currently available therapeutical options are sufficient to successfully control SHPT and maintain calcium and phosphate levels in both G3a‑G3b and G4 CKD patients.
In contrast, the nephrologists disagreed with the following:
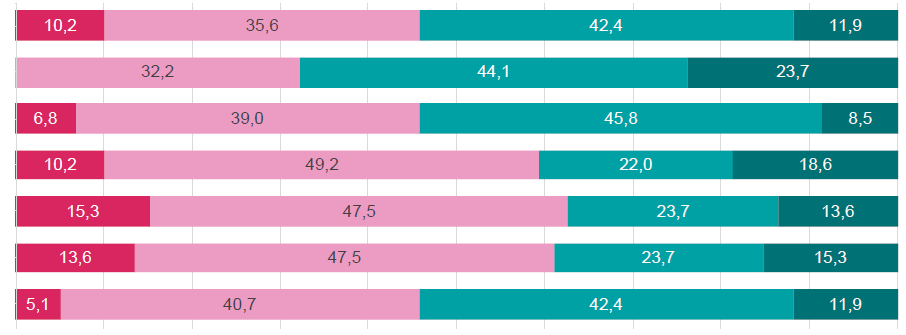
The participants did not reach an agreement concerning the incidence of SHPT in adult patients with CKD G3a‑G3b. Accordingly, the responses revealed heterogeneity regarding the management of SHPT in their clinical practice, stemming from undefined PTH levels and sub‑optimal therapeutic strategies. More, participants were also not unanimous on their prescription of vitamin D‑based approaches (native vitamin D supplements or active vitamin D/analogues) for G3a‑G3b and G4 CKD patients nor the estimation of G3a‑G4 uremic patients under active vitamin D/analogs treatment (<10%).
DISCUSSION
This study assessed the agreement level on the diagnosis and treatment of SHPT in CKD patients (stages G3‑G4) among members of the Portuguese Society of Nephrology (SPN), using a one‑round‑only Delphi‑adapted panel of 17 statements.
No statement reached a consensus, indicating heterogeneity in clinical practice and the existence of unclear therapeutic guidelines regarding SHPT diagnosis and management in pre‑dialysis uremic patients. A qualified majority was obtained in 59% (10 out of 17) of the statements; specifically, a qualified majority of agreement was obtained in 47% (8 out of 17) of the statements and of disagreement in 12% (2 out of 17) of the statements.
The nephrologists agree that SHPT is frequently detected in G4 CKD patients, in line with a reported incidence of 80% in stage G4 CKD patients.4 Serum levels of calcium, phosphate, and PTH should be monitored at intervals of every 6‑12 months for G3a‑G3b CKD and of 3‑6 months for CKD G4, whereas PTH should be based on baseline level and CKD progression for G3a‑G3b CKD and monitored every 6‑12 months for G4 CKD. This is in line with KDIGO 2017 guidelines’ update for SHPT on CKD.8
Most nephrologists agreed to use native vitamin D to correct its insufficiency or deficiency and then active vitamin D/analogs to control subsequent SHPT. Concordantly, National Kidney Foundation‑Kidney Disease Outcomes Quality Initiative (NKF‑KDOQI) 2003 guidelines recommend native vitamin D supplementation in patients with stage 3 to 4 CKD with 25(OH)D levels <30 ng/mL and active vitamin D analogues if SHPT (PTH >300 pg/mL) develops.11 This was further corroborated by the KDIGO 2017 guidelines, where the routinely use of vitamin D analogs was not suggested for adult patients with non‑dialysis G3a‑G5 CKD (due to the failure of the PRI‑MO and OPERA studies to demonstrate clear clinical benefits in CKD patients with moderately increased PTH level30,31), thus being only recommended for patients with CKD G4-G5 with severe and progressive hyperparathyroidism.8 Approximately 75% of the nephrologists agreed with the estimate of approximately <70% of adult patients with CKD G3a‑G4 receiving native vitamin D supplementation, which is in agreement with real‑world data.32 The participants also demonstrated a high agreement level regarding the efficacy of currently available therapeutical approaches to control SHPT and maintain calcium and phosphate levels in G3a‑G3b (91.5% of agreement) and G4 (78% of agreement) CKD.
When inquired about treating G4 CKD patients with SHPT using only native vitamin D, the physicians majorly disagreed (72.9%). Accordingly, previous NKF‑KDOQI guidelines recommend active vitamin D for SHPT in CKD. However, routine use of active vitamin D/analogs is not recommended anymore for pre‑dialysis G3a‑G5 CKD due to the risk of hypercalcemia, being reserved only for severe SHPT in G4‑G5 CKD patients,8 not being fully established that supplementation with native vitamin D (ergocalciferol or cholecalciferol) is indeed effective.11
Most nephrologists disagreed that in their clinical practice, the majority of non‑dialysis adult patients present serum total 25(OH)D >50 ng/mL and controlled levels of PTH (or, at least, without increasing it) only with native vitamin D. These results reflect the literature, as current clinical data in patients with G3‑G5 CKD demonstrate nutritional vitamin D to inconsistently increase serum 25(OH)D levels above 50 ng/mL and decrease PTH levels. In contrast, reduced PTH with active vitamin D analogues is accompanied by undesired hyperphosphatemia and hypercalcemia. This therapeutic gap opens the possibility to explore the novel ERC molecule, which is positioned between nutritional vitamin D and active vitamin D analogues therapies22,23- the extent of increase of 25(OH)D levels effectively suppresses PTH, under safe and minimal changes in serum phosphorus and calcium.
A considerable heterogeneity among the Portuguese nephrologists’ opinions was disclosed as nearly 42% of the statements failed to gather consensus or a qualified majority. Almost half of the physicians disagreed that SHPT is a frequent and critical complication of G3a‑G3b CKD, which contradicts the mounting reports of high prevalence rates of 40% and 80% in G3 and G4 CKD patients, respectively.4,5More than half of the nephrologists (68%) that responded to the questionnaire were permissive regarding PTH in G3a‑G4 CKD patients, as PTH optimal levels are unknown. Diagnostic and therapeutic strategies for SHPT in CKD are frequently suboptimal due to the lack of reliable reference markers as target PTH levels. To minimize this, KDIGO 2017 recommends that therapeutic decisions be based on trends rather than on a single laboratory value, considering all available markers (PTH, calcium, phosphorus, and vitamin D).8 Moreover, PTH monitoring should be based on baseline level and CKD progression for G3a‑G3b and every 6‑12 months for G4 CKD.8
Participants were also divided (agree: 54%; disagree: 46%) when asked whether they consider the available therapeutical options for SHPT sub‑optimal for G3a‑G4 CKD. Although effective, current therapeutical strategies still possess relevant side effects (hypo/hypercalcemia, hyperphosphatemia) and limited capacity to improve significant clinical outcomes. There is, therefore, an urgent need for new and improved SHPT treatments in G3‑G4 CKD patients.33
Despite not achieving a qualified majority, more than 50% of the participants disagreed with treating SPTH‑associated vitamin D insufficiency with only native vitamin D (disagree: 59%) or only active vitamin D/analogs (disagree: 63%) on G3a‑G3b CKD patients. These results depict the difficulties in the clinical practice due to the lack of precise data, the scarce therapeutic agents currently licensed for use in non‑dialysis CKD patients with SHPT and whose optimal management remains controversial, and the urgent need for novel therapeutic approaches.
Most participants disagree with using only active vitamin D/analogs for G4 CKD patients with SHPT, which is in agreement with the KDIGO recommendations of reserving active vitamin D/analogs for patients with G4‑G5 CKD with severe and progressive SHPT only.8 Accordingly, the participants were also divided with the estimate that less than 10% of the G3a‑G4 CKD patients currently receive active vitamin D/analogs. Though this could reflect the recommendation of non‑generalized use of vitamin D analogues due to safety issues, the real‑world data demonstrate the opposite, with reports of 53%‑62% of G3‑G4 CKD patients on active vitamin D/analogues treatment.32
In Delphi studies, a range of 10‑15 respondents is generally considered to be sufficient to enable consensus to be achieved, with larger sample sizes resulting in potentially low response rates.34‑39Therefore, we believe that a number of 59 responding nephrologists is adequate and sufficient for drawing important conclusions regarding the diagnosis and treatment of SHPT in CKD patients (stages G3‑G4) in Portugal. The authors acknowledge that although it might have been interesting to characterize the nephrologists who responded to the survey, for example, in terms of years of clinical practice, this was not possible due to the anonymity of the process. Furthermore, it would be appealing to become acquainted with international clinical practice and compare it with the results observed herein and also the compliance with KDI‑GO guidelines in the pre‑dialysis environment.
CONCLUSION
This study disclosed a discrete level of agreement on the current strategies to monitor and control the progression of SHPT and its surrogate markers (PTH, calcium, phosphate, and vitamin D) in G3a‑G4 CKD patients. Still, optimal management of SHPT in non‑dialysis CKD patients remains challenging, as acknowledged by international committees, which directly impacts healthcare providers. The prevalence of SHPT in G3a‑G3b CKD patients is not consensual, eventually leading to sub‑diagnosis and sub‑treatment. Furthermore, the observed discordance among nephrologists regarding the efficacy and safety of currently available therapeutical options for SHPT and SHPT‑associated hypovitaminosis D may indicate an urgent need for high‑quality clinical trials determining the effect of vitamin D supplementation on the SHPT clinical burden. Conclusively, additional scientific and clinical research to define target levels and unravel novel surrogate markers and treatment options should be pursued to foster the definition of consensual guidelines and standards of care.
TAKE HOME MESSAGES
Strategies to monitor and control the progression of SHPT and its surrogate markers (PTH, calcium, phosphate, and vitamin D) in G3a‑G4 CKD patients are not consensual among nephrologists.
This Delphi‑like panel demonstrated a modest level of agreement between the Portuguese nephrologists regarding the optimal approaches for optimal management of SHPT in non‑dialysis CKD patients.
The discrete level of agreement between nephrologists reflects the urgent need to draw consensual guidelines for treatment and management of SHPT in pre‑dialysis CKD, and the pressing need to develop novel therapeutic approaches.