INTRODUCTION
Chronic kidney disease (CKD) is a leading cause of morbidity and mortality in both developed and developing countries. Studies have consistently shown that African descendants are at increased risk of CKD occurrence and progression to end‑stage renal disease (ESRD),1 with CKD being at least 3‑4 times more frequent in Africa than in higher income countries.2‑4Nevertheless, the burden of CKD and ESRD in African continent, more specifically sub‑Saharan Africa, is still largely conjectural as statistics from most of those countries are unavailable due to a lack of registries.3,5
Despite the higher prevalence, access to treatment of CKD is restricted in those areas,3,6especially when the disease achieves an advanced stage, with the need for renal replacement therapy (RRT). Africa contributes to <10% of the total RRT patients worldwide, and this is mainly due to its associated high costs.7,8Hemodialysis (HD) is the most common renal replacement therapy (RRT) modality in Africa although few countries enjoy reimbursement from the government to fund it.4,8,9
For that matter, international healthcare agreements that allow these patients to initiate HD elsewhere were created.10 Patients from African Countries of Portuguese Official Language (ACPOL) can be referred to Portuguese hospitals of the National Health System in situations of proven lack of capacity or ability of the public units of HD in those countries, namely, when there are no HD units in their country, or in case of access failure or low dialytic efficacy. Nevertheless, referral is often late, in many cases in advanced stages of renal disease with the need of urgent HD, and some patients resort coming to Portugal through their own means.11,12
The purpose of this analysis was to describe the clinical characteristics and outcomes of patients from ACPOL who integrated the HD program of a tertiary hospital in Portugal.
MATERIAL AND METHODS
This study was a retrospective analysis of 126 African patients who integrated the regular hemodialysis program at the HD unit of Centro Hospitalar Universitário Lisboa Norte (CHULN) in Lisbon, Portugal, between January of 2013 and December of 2019. The Ethical Committee approved this study, in agreement with institutional guidelines. Informed consent was waived, given the retrospective and noninterventional nature of the study.
Participants
We selected as eligible all adult patients (≥18 years of age) with CKD who were referred from the ACPOL and integrated into the regular HD program.
Variables and Outcomes
Data was obtained from individual papers and electronic clinical records. The following variables were collected: demographic characteristics (age, gender, race, born country); CKD etiology; Comorbidities [diabetes, hypertension, cerebrovascular disease (CVD), heart failure, ischemic cardiopathy, chronic obstructive pulmonary disease (COPD), chronic liver disease, peripheral artery disease, rheumatological disease and/or active malignancy]; motive of referral to Portugal (RRT requirement, including urgent RRT, other health condition in a patient with CKD on HD, and patient’s option; and vascular access (VA) dysfunction); viral serologies (human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV); laboratory values at arrival to Portugal [hemoglobin (Hb), serum creatinine (SCr), estimated glomerular filtration rate (eGFR), serum urea, serum albumin, serum ferritin, serum parathyroid hormone (PTH), C‑reactive protein (CRP)]; HD vascular access at admission and at the end of the follow‑up [central venous catheter (CVC), arteriovenous fistula (AVF), arteriovenous graft (AVG)]; RRT prior to arrival to Portugal. We also analyzed the rate of patients transferred to peritoneal dialysis and/or who underwent kidney transplantation, the time to kidney transplantation when appliable and the mortality rate during follow‑up. Data collection was made in January/2021.
Definitions
The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‑EPI) creatinine equation.13 CKD was defined as eGFR lower than 60 mL/min/1.73 m2. Diabetes was defined in accordance with American Diabetes Association Guidelines.14 Arterial hypertension was diagnosed according to European Society of Cardiology and European Society of Hypertension Guidelines.15 Cerebrovascular disease was defined based on prior history of stroke, carotid, vertebral or intracranial stenosis, aneurysms or vascular malformations.16 Uncontrolled anemia in the context of CKD was defined as an hemoglobin value < 10 g/dL according to the Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines.17 Vascular access failure was defined as recurrent loss of a permanent VA for HD, namely recurrent AVF or AVG thrombosis and/ or recurrent CVC dysfunction, requiring intervention.18
RESULTS
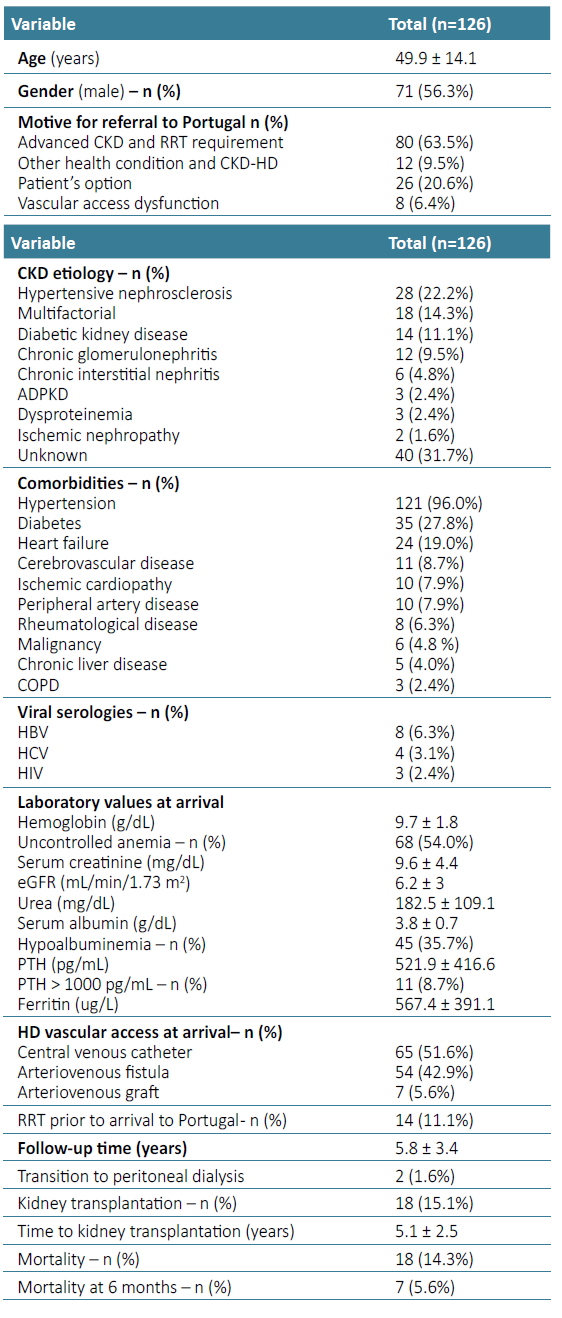
A total of 126 African patients underwent chronic hemodialysis at the HD unit of Centro Hospitalar Universitário Lisboa Norte. Baseline characteristics of this cohort are described in Table 1. Mean age was 49.9 ± 14.1 years, and the majority was male (n= 71, 53.6%). Twenty‑one patients came from Angola (16.7%), 53 from Cape Verde (42.0%), 23 from Guinea‑Bissau (18.3%), 20 from Saint Thomas and Prince (15.9%), and 9 from Mozambique (7.1%) (Fig. 1).
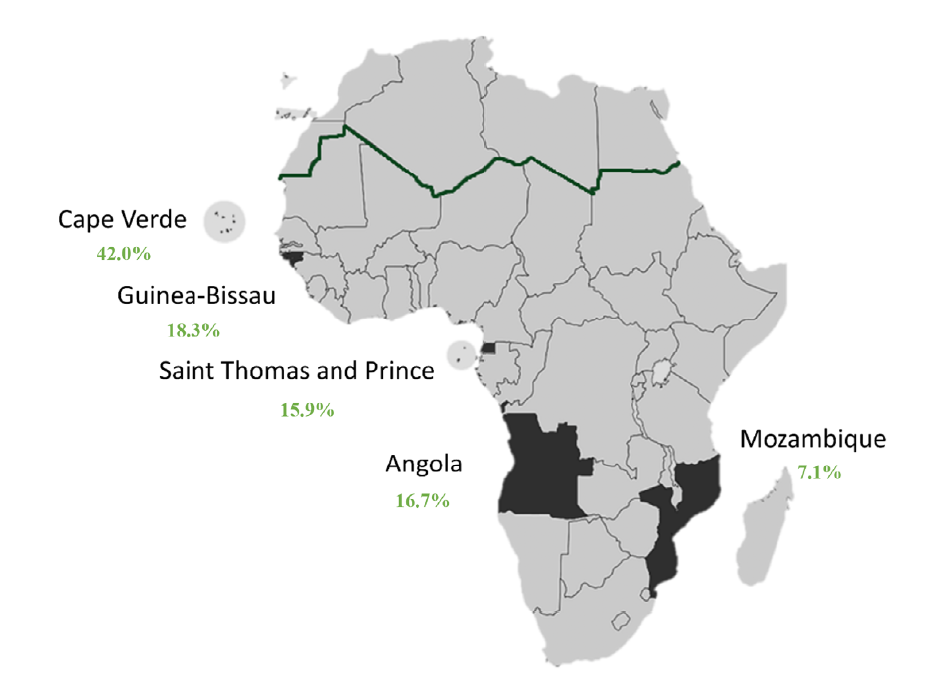
Figure 1. Map of Africa with ACPOL highlighted. Rate of patients from each ACPOL in green. Sub‑Saharan Africa below the dark green line.
Motives for referral to Portugal were advanced CKD and urgent RRT (n= 80, 63.5%), other health condition in CKD on HD patients (n=12, 9.5%) and VA dysfunction (n=8, 6.4%). Twenty‑six patients (20.6%) were on RRT and opted to come to Portugal in search of better HD and CKD care. In 31.7%, CKD etiology was undetermined (n=40), followed by hypertensive nephrosclerosis in 22.2% (n=28), multifactorial in 14.3% (n=18), diabetic kidney disease in 11.1% (n=14), chronic glomerulonephritis in 9.5% (n=12) with 4 cases related to viral infection (HIV in 3 patients and HBV in 1 patient), chronic interstitial nephritis in 4.8% (n=6), autosomal dominant polycystic kidney disease (ADPKD) in 2.4% (n=3), dysproteinemia in 2.4% (n=3), and ischemic nephropathy in 1.6% (n=2).
There was a large prevalence of hypertension (96.0%, n=121) and diabetes (27.8%, n=35). Heart failure was present in 19.0% (n=24), CVD in 8.7% (n=11), ischemic cardiopathy in 7.9% (n=10), peripheral artery disease in 7.9% (n=10). Also, 4.8% of patients had active malignancy (n=6). Regarding laboratory parameters, patients who integrated the HD program in our unit had mean sCr 9.6 ± 4.4 mg/dL, urea 182.5 ± 109.1 mg/dL, Hb 9.7 ± 1.8 g/dL, with 54.0% having uncontrolled anemia (n=68), ferritin 567.4 ± 391.1 ug/L, serum albumin 3.8 ± 0.7 g/dL, with 35.7% having hypoalbuminemia (n=45), and PTH 521.9 ± 416.6 pg/mL. There were no statistically significant differences concerning country of origin, although hypoalbuminemia was more frequent in patients from Saint Thomas and Prince (50%) and Angola (42.9%). Laboratory parameters according to motive for referral to our center are represented in Table 2.
Table 2. Laboratory parameters at arrival of patients from ACPOL according to motive for referral to our center.
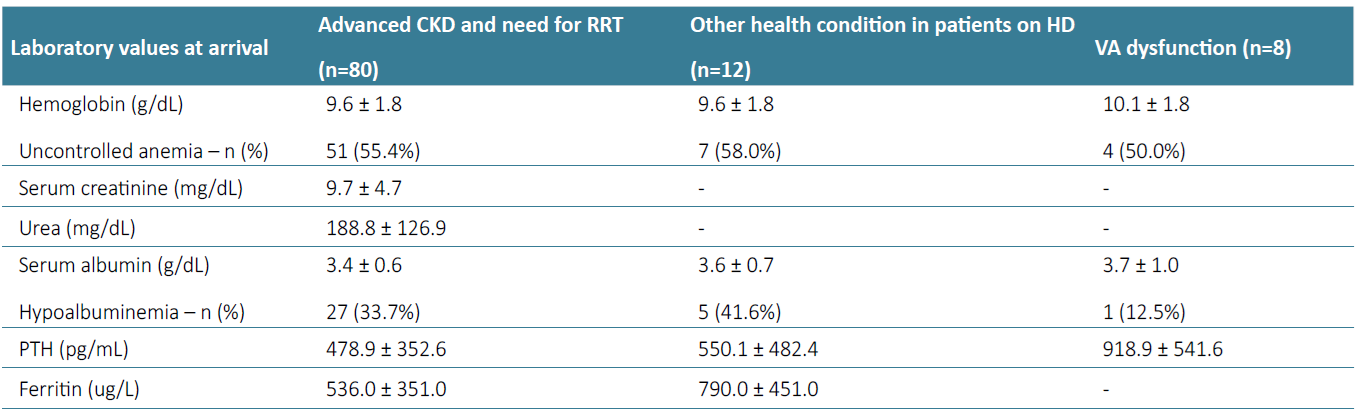
Advanced CKD and need for urgent RRT
Regarding laboratory parameters, patients with advanced CKD and need for urgent RRT who integrated our HD program (n=80) had mean sCr 9.7 ± 4.7 mg/dL, urea 188.8 ± 126.9 mg/dL, Hb 9.6 ± 1.8 g/dL, with 55.0% having uncontrolled anemia (n=44), ferritin 536.0 ± 351.0 ug/L, serum albumin 3.4 ± 0.6 g/dL, with 33.7% having hypoalbuminemia (n=27), and PTH 478.9± 352.6 pg/mL. All patients started HD with a CVC. Of the 80 patients who came due to advanced CKD and urgent RRT, 55.0%(n=44) were submitted to AVF and AVG construction during follow‑up (n=39 and n=5, respectively).
Other health condition in patients on HD
Patients who came due to other health condition and were already on regular HD in their country (n=12), had mean Hb 9.6 ± 1.8 g/dL, with 58.0% having uncontrolled anemia (n=7), ferritin 790 ± 451 ug/L, serum albumin 3.6 ± 0.7 g/dL, with 41.6% having hypoalbuminemia (n=5), and PTH 550.1 ± 482.4 pg/mL. In this group of patients, five already had an AVF and the remaining 7 had a CVC as VA for HD. During follow‑up in our center, three patients were submitted to construction of an AVF and 1 of an AVG. Three patients still had a CVC at the end of follow‑up.
VA dysfunction
Patients that were referred due to VA dysfunction (n=8) presented mean Hb 10.1 ± 1.8 g/dL, with 50% having uncontrolled anemia (n=4), serum albumin 3.7 ± 1.0 g/dL, with 12.5% having hypoalbuminemia (n=1) and PTH 918.9 ± 541.6 pg/mL. Patients who came to Portugal due to VA dysfunction were mainly from Cape Verde (n=4), Angola (n=3), and one patient from Guinea‑Bissau (doing previously HD in Angola). Patients who came to our center due to VA dysfunction presented a history of end‑stage VA failure in 37.5% (n=3), all having superior vena cava syndrome and two with a VA in an atypical location (CVC in femoral vein and a femoral‑femoral AVG). CVC was the VA at arrival in 50.0% (n=4), AVG in 25.0% (n=2) and AVF in 25.0% (n=2). During follow‑up, two patients underwent AVF construction, one patient was submitted to super urgent renal transplantation and one patient transited to PD.
FOLLOW‑UP
The mean follow‑up time was 5.8 ± 3.4 years. One point six per cent of the 126 patients transited to peritoneal dialysis (n=2) and 15.1% were submitted to renal transplantation (n=18); the time to kidney transplantation was 5.1 ± 2.5 years. In the group of patients with VA dysfunction at admission (n=8), 2 patients underwent renal transplantation and 2 are on active waiting list. The mortality rate during follow‑up was 14.3% (n=18), with 5.6% of patients dying within the first 6 months of follow‑up (n=7). Five patients died from sepsis (four of them had a CVC as VA for HD), one patient due to advanced lung carcinoma, one from sudden death. Cause of death was undetermined in 10 patients. At last, one patient from Saint Thomas and Prince, with ESRD on regular hemodialysis program, wished to return to her born country, aware of the fatal outcome arising from that decision, since HD is not an option there. This patient presented several comorbidities and multiple hospital admissions, and her final wish was to be with her family.
Characteristics According to Country of Origin
Patients’ characteristics according to country of origin are presented in Table 3.
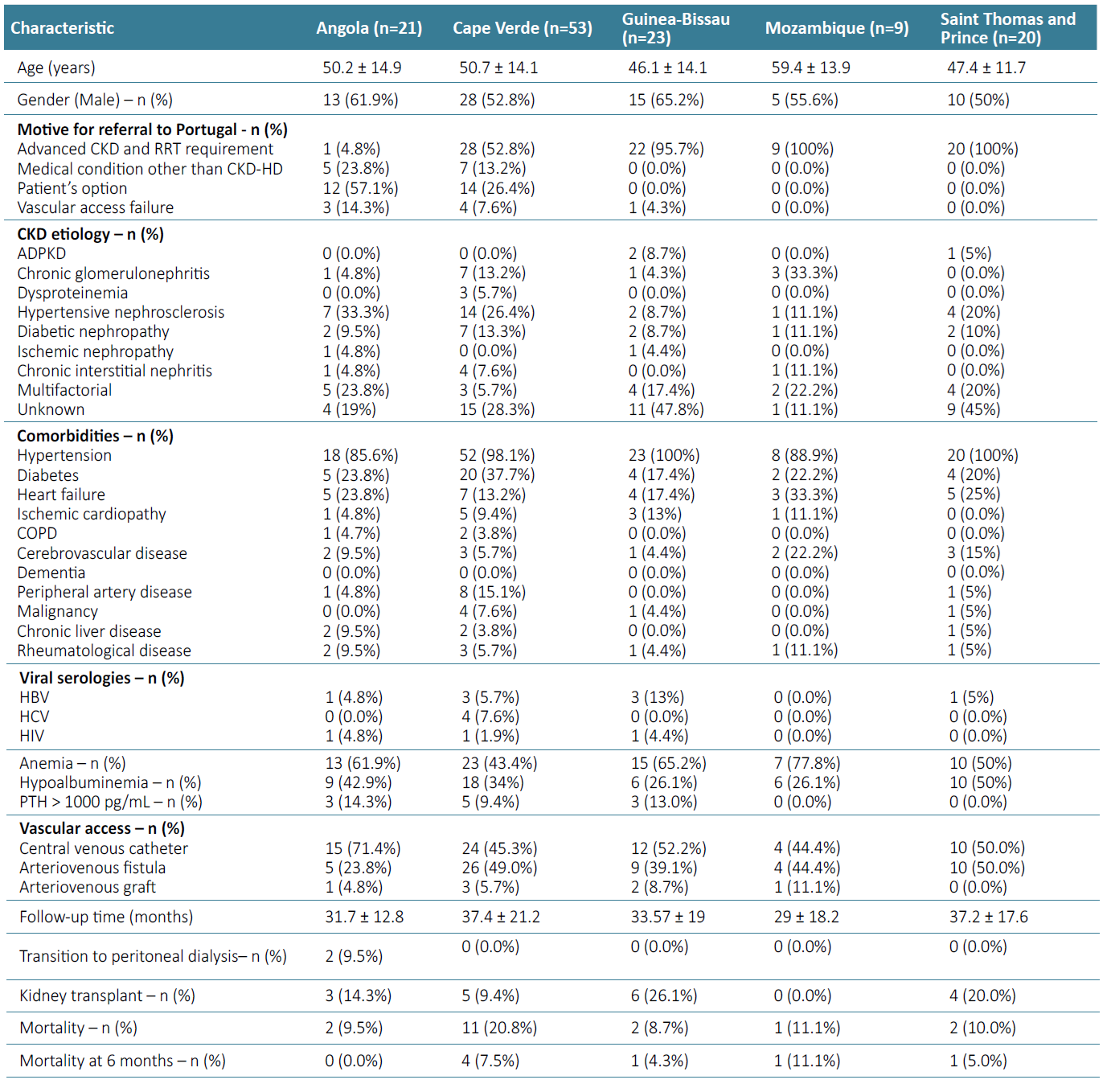
Patients arriving from Angola had a mean age of 50.2 ± 14.9 years, and the most common CKD etiology was hypertension (33.3%, n=7). Motive for coming to Portugal was mainly patient’s option (57.1%, n=12), followed by a medical condition other than CKD‑HD (23.8%, n=5), VA failure (n=3, 14.3%) and one patient came due to advanced CKD in need of HD. Five patients (23.8%) had a history of primary failure of vascular access and 2 presented multiple vascular access failure (9.5%), with these cases being the most serious and worrisome. During follow‑up, 2 patients transited to peritoneal dialysis (9.5%) and 3 were submitted to kidney transplantation (14.3%). Mortality rate was 9.5% (n=2) during follow‑up. Patients arriving from Cape Verde had a mean age of 50.7 ± 14.1 years, almost all patients had hypertension (98.1%, n=52), and diabetes was present in 37.7% (n=20). Motive for referral to Portugal was advanced CKD and urgent RRT in 52.8% (n=28) and a health condition other than CKD‑HD in 13.2% (n=7).
Fourteen patients (26.4%) came by option and four patients (7.5%) due to VA dysfunction. During follow‑up, 5 patients were submitted to kidney transplantation (9.4%) and mortality rate was 20.8% (n=11), with 7.5% dying at 6 months (n=4). Patients arriving from Guinea‑Bissau had a mean age of 46.1 ± 14.1 years, and most patients had undetermined cause of CKD (47.8%, n=11). Motive for referral to Portugal was RRT requirement in 95.7% (n=22) and vascular access dysfunction in one patient that was on HD in Angola. All patients had hypertension. Four patients presented multiple vascular access failure (17.4%). During follow‑up, 26.1% were submitted to kidney transplantation (n=6) and mortality rate was 8.7% (n=2). Patients arriving from Mozambique had a mean age of 59.4 ± 13.9 years, and chronic glomerulonephritis was the CKD cause in 33.3% (n=3).
Motive for referral to Portugal was advanced CKD and urgent RRT requirement in all patients. Hypertension was present in 88.9% of patients. One patient died (11.1%) within the first 6 months of HD. None of the patients were submitted to kidney transplantation or transitioned to peritoneal dialysis. Patients arriving from Saint Thomas and Prince had a mean age of 47.4 ± 11.7 years. Motive for referral to Portugal was advanced CKD and urgent RRT in all patients. CKD etiology was unknown in 45% of the patients (n=9) and all had hypertension. During follow‑up, 4 patients were submitted to kidney transplantation (20%) and 2 transitioned to peritoneal dialysis (10%). Mortality rate was 5.0% (n=1).
DISCUSSION
In this study, we report a significant number of patients from Angola, Cape Verde, Guinea‑Bissau, Saint Thomas and Prince and Mozambique that underwent hemodialysis at our center. The main reasons were advanced CKD and RRT requirement, a medical condition other than CKD‑HD and VA failure. We also report a significant incidence of patients who opted to come to Portugal in search of improved CKD and HD care. CKD, specifically ESRD, is largely a disease of the young and middle‑aged (20‑50 years) in sub‑Saharan Africa, a sharp contrast to the older age patients in the high‑income countries. The mean age of ESRD patients in a Nigerian study was 41.5 ± 16.2 years,7 while European Renal Association (ERA) Registry of 2019 presented a median age at the start of RRT of 67.9 years and in prevalent European patients of 60.5 years.19 Also, the Portuguese registry of Dialysis and Transplantation of the same year revealed that more than 65% of the prevalent patients on hemodialysis were >65 years old.20 The younger age reported in our cohort (49.9 ± 14.1 years) can be explained by several factors, such as the insufficient identification of patients at risk, the limited capacity of healthcare professionals and facilities to implement measures of CKD prevention and follow‑up, the scarce kidney disease awareness in the community, and the low availability of RRT in those countries.3 These differences accentuate the necessity of creating and improving resources to the population in need of healthcare in each country. Another important difference, when comparing with Euro‑ pean registries, is the pattern and etiology of renal diseases in Africa.8,19,21
In our cohort, an important rate of patients presented ESRD of undetermined cause, followed by hypertensive nephrosclerosis, diabetic kidney disease, chronic glomerulonephritis related to viral infections and chronic interstitial nephritis. Diabetic nephropathy is the leading cause of ESRD worldwide, while in the African continent, hypertension, glomerulopathies and infections such as leishmaniasis, schistosomiasis, HIV and other viral infections account to a disproportionately large number of cases, which also justifies the younger age of these patients.3,5,22,23Also, African continent presents the highest prevalence of HIV‑1 infection and it is known the robust association between Apolipoprotein L1 gene variants in African descendants, which results in an increased risk for focal segmental glomerulosclerosis and HIV‑associated nephropathy.24 Advanced CKD with RRT requirement and medical conditions other than CKD‑HD were the main reasons of referral of ACPOL patients to Portugal in our study (63.5% and 9.5%, respectively) followed by VA failure (6.4%).
The Global Kidney Health Atlas Survey concluded that kidney replacement treatments availability, access, and quality are very limited in African countries; government funding for kidney care is low as well as the number of nephrologists in proportion with the amount of ESKD patients.25 Given the dismal prognosis of an ESRD patient, outcomes are divided into two groups: those who cannot afford RRT and are thus placed on conservative management, dying in the first few weeks to one year after ESRD diagnosis, and those who can financially sustain the treatment. However, a study demonstrated that only about 4% could afford the treatment beyond 12 weeks, and 90% were unable to continue dialysis after the first month.3 This is probably why many patients decide to come to Portugal through their own means, and stay here, as occurred in 20.6% of these patients, sometimes in an illegal situation. Therefore, it is important to implement strategies to provide effective hemodialysis in these countries so that patients can have access to better care.
All of the African patients in our cohort started HD with a CVC and several presented end stage VA failure. A recent study revealed that in East Africa, most patients end up needing dialysis in a sudden way, as advanced CKD follow‑up is generally nonexistent, and VA planning is not a priority.26 Furthermore, cultural beliefs and practices seemed to influence patient compliance to the recommended care in preventing infection or other complications.26
No accurate data about medical services and kidney care in Africa is available.9 According to the International Society of Nephrology Global Health Kidney Health Atlas last published data, the burden of hemodialysis in Angola was 23.6 per million population and Mozambique 0.92 per million population. Cape Verde, Saint Thomas and Prince, Guinea‑Bissau presented no data.25 Regarding kidney replacement therapy in Africa, only 18 countries reported the ability to provide HD 3 times per week for 3‑4 hours per session, which was lowest in West Africa (n=2). Moreover, the majority of patients started dialysis with temporary and tunneled catheters.8 In our study, all of the patients started hemodialysis with a catheter, and we report a high prevalence of vascular access problems, many of them with multiple prior CVCs. This highlights the importance of medical education and training regarding the early planning of a definitive VA for hemodialysis. Thus, although cooperation protocols are a way to significantly improve patients’ outcomes, investment in material resources and medical expertise is urgently needed in most African countries. Portuguese vascular surgeons have been available to travel to Angola and Cape Verde to teach and train the local vascular surgery teams. Since hemodialysis programs were successfully implemented in Cape Verde in 2014, these patients no longer need urgent evacuation to other countries for this treatment. This represents a positive sign of this type of interaction between countries and one which can be replicated in others. In Guinea‑Bissau and Saint Thomas and Prince there are no HD clinics, and hundreds of patients from these countries maintain hemodialysis in Portugal. In Mozambique, hemodialysis is only available in two small units.12,27
The development of HD clinics in these countries is essential to improve health care and patient outcomes. Treatment of CKD related complications should also be crucial in the management of these patients. Our results are in line with literature data revealing that anemia and mineral bone disease treatment is far from what is recommended in guidelines, due to inadequate patient monitoring, lack of awareness among both patients and physicians, but also due to poor availability of treatment options.8,28,30Mortality in African patients on dialysis has been scarcely reported. A study involving 661 patients on HD in Cameroon revealed a mortality rate of 26.8% (n= 17) at 12 months and 44.9% during a 10‑year follow‑up.4 In a study of incident dialysis cases in Ghana, thirty‑two per cent died within 90 days of starting dialysis and in incident dialysis patients in Ethiopia, the death rate was 23.0% at 90 days.4 The causes of death included uremia, volume overload, uncontrolled hypertension, lack of vascular access, heart failure, stroke, and infections.3 In our study, mortality rate was 14.3% (n=18) in 7 years, with 39.0% of these dying within the first 6 months of follow‑up (n=7). Late presentation (or referral) with CKD and affordability are often cited as major drivers of the high early mortality, as well as lack of timely access to pre‑ESRD nephrology care, ESRD education and support services, treatment of complications in advanced CKD, and timely placement of a permanent vascular access.3 Investing and promoting CKD complications’ treatment in ACPOL is essential to improve these patients’ quality of life and survival. In order to identify the major gaps and to find adequate solutions, it is also important to improve African countries’ healthcare care registries.
Finally, once these patients are brought to Portugal, it is of paramount importance to promote their social and professional integration, in order to contribute to the financial investment in their treatments. Some limitations should be considered when interpreting this study. This is a retrospective single centre study in Portugal, which limits the generalization of the results. Additionally, as information is scant concerning these countries, some data was only gathered through websites. However, we must also highlight the major strengths as this is the first study describing the clinical characteristics and outcomes of HD patients referred from the ACPOL, so it presents additional and paramount data about CKD, ESRD and RRT outcomes in African patients.
CONCLUSION
This is the first study presenting the clinical characteristics and outcomes of ACPOL hemodialysis patients in Portugal. Most of these patients come due to a cooperation protocol between countries, in need of RRT and due to VA dysfunction. Still, a significant proportion of CKD patients come to Portugal on their own. All of these patients started HD with a CVC, which is widely known to be associated with mortality. With the cooperation protocol, Portugal provides with RRT, treatment of the CKD complications, adequate VA care, possibility of peritoneal dialysis and kidney trans‑ plantation to ACPOL patients. Nevertheless, the corner‑stone to improve these patients’ quality of life and survival is a proper investment in African countries’ healthcare, financial and educational, namely regarding CKD management and ESRD.