INTRODUCTION
Renal artery thrombosis is a rare and overlooked cause of kidney failure.1 It is more prevalent between the age of 30 and 50 years, with no gender predominance.2,3
Most cases of renal artery thrombosis are due to thromboemboli originated in the heart, (mainly related to atrial fibrillation and infective endocarditis), or in aorta (after endovascular or surgical interventions, where injury to the endothelium or dislodging an atherosclerotic plaque can occur).4 In-situ thrombosis of renal artery is uncommon, and the most frequent causes are atherosclerotic lesions of the renal artery and blunt abdominal trauma. Other described promotor conditions are renal interventions, coagulation disorders, vasculitis, connective tissue diseases, pregnancy, renal transplantation, oral contraceptives, polycythemia vera, cocaine injection, nephrotic syndrome, renovascular hypertension, Ehlers‑Danlos syndrome. Spontaneous renal artery thrombosis, without any identified etiology nor risk factor, is extremely rare.2 Clinical presentation of renal artery thrombosis most often includes abdominal and/or flank pain, which is typically persistent and can mimic symptoms of nephrolithiasis,
pyelonephritis or other intra‑abdominal condition. Other clinical manifestations are hematuria, fever, nausea, and vomiting.1,4Renal artery thrombosis leading to partial obstruction and ischemia without infarction may not cause any symptoms, but eventually progresses to renal dysfunction as decreased perfusion persists.3
The emergence of contrast‑enhanced computed tomography (CT) scans and other imaging modalities have allowed the detection of incidental cases of renal infarction. On that account, there is probably a relevant number of underreported cases, explained by the nonspecific clinical presentation. The early recognition of this entity is essential to initiate revascularization therapy promptly and improve renal outcomes.4
Herein, we present a rare case of bilateral renal artery thrombosis as a cause of severe acute kidney injury (AKI), with a favorable outcome under apixaban.
CASE REPORT
A 61‑year‑old Caucasian female presented to the emergency department due to recent and persistent history of nausea and vomiting. She mentioned a 6‑month history of melenas, with episodes of constipation and diarrhea, weight loss of 10 kg and she noted a reduction of urine output in the previous days. Patient’s medical history was remarkable for urticaria syndrome with previous exposure to long term systemic therapy with corticosteroids which led to secondary osteoporotic fractures; she also presented past history of smoking of 4 pack‑year, having stopped one year before, and mild severe acute respiratory syndrome coronavirus type 2 (SARS‑CoV‑2) infection two months before, manifested as cough and coryza, with no need for oxygen therapy or any specific treatment.
The patient also mentioned an episode of acute right‑sided flank and back pain three months before the current clinical condition, with no other symptoms, which resolved spontaneously. A renal ultrasound was performed at that time, and it showed normal sized symmetric kidneys with regular contours, normal parenchymal/central‑complex differentiation with conserved cortical index, with no hydronephrosis and/or calculi. Lab work documented elevated serum creatinine (sCr) (7.5 mg/dL) and microcytic hypochromic anemia (hemoglobin 7.2 g/dL), with a positive fecal occult blood test in 3 samples. The patient’s renal function was normal three months before (sCr 0.7 mg/dL).
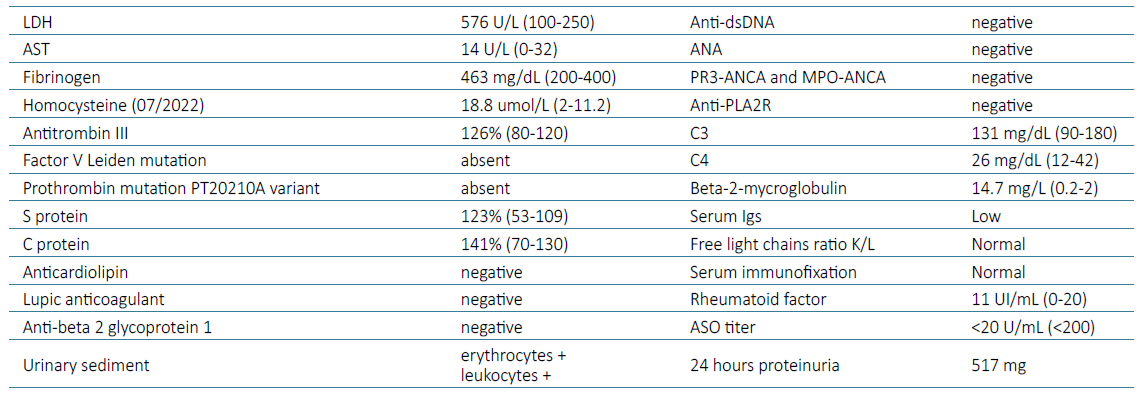
The patient was admitted for further investigation of severe AKI and upper gastrointestinal bleeding. At admission, a renal ultrasound was performed, and documented right kidney atrophy, with 6 cm bipolar axis, and left kidney with 9.5 cm of bipolar axis and reduction of the parenchymal width proportional to the reduced kidney dimension, with preserved sinusal parenchyma; no hydronephrosis or peri‑renal fluid present. These findings led to an abdominal‑pelvic computed tomography with intra‑venous contrast (five days after admission) that revealed right renal artery occlusion with an atrophic ipsilateral kidney (5 cm) as well as left renal artery occlusion with a spontaneous hyperdense thrombus associated with a left kidney size of 7 cm, with contrast enhancement maintained only on its superior pole, in relation with a permeable accessory renal artery (Fig. 1). The case was discussed with Vascular Surgery who deliberated that there was no indication for surgical or endovascular intervention, since the right kidney had already evidence of atrophy and the left arterial renal thrombosis occurred at least 5 days before and was deemed irreversible. Laboratory investigation is presented in Table 1. Prothrombin time, partial thromboplastin time and platelet counts were normal. Investigation of hereditary thrombophilia was negative (Table 1), and there was no family history of arterial or venous ischemic disease.
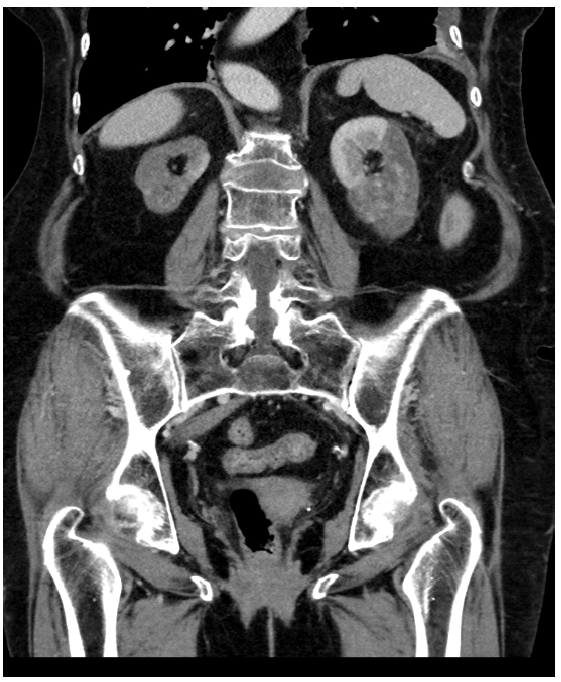
Figure 1. Axial abdominal‑pelvic computed tomography scan enhanced with intravenous contrast injection documenting signs of acute left renal artery thrombosis, with contrast enhancement of the superior pole in association with permeable accessory renal artery, and non‑recent right renal artery thrombosis with right kidney atrophy.
The echocardiogram was normal with no evidence of clots in the cardiac chambers. The thoracic‑abdominal‑pelvic CT scan presented no signs of neoplasia. Regarding investigation of melenas and iron deficiency anemia, upper digestive endoscopy was performed and documented a giant hiatal hernia, duodenitis and Forrest III duodenal ulcers (bulb and D2 with edematous mucosa with intense hyperemia foci, and 2 ulcers). Colonoscopy was normal. The patient was initiated on proton pump inhibitor and, afterwards, anticoagulated with enoxaparin 60 mg/day, but evolved with persistent renal dysfunction with urea 230 mg/dL and sCr 7.8 mg/dL, oliguria with volume overload and metabolic acidosis. Hemodialysis was initiated and the patient was discharged, anticoagulated with apixaban 2.5 mg twice a day, with indication to maintain ad eternum given the undetermined cause of the thrombosis. Antiplatelet therapy was not initiated along with apixaban, due to the bleeding risk. In the Outpatient HD Clinic, hemodialysis protocol consisted of 3 sessions/week, 4 hours/session, high flux hemodialysis using the Nephral dialyzer with the AN69‑ST acetate‑free membrane, due to a previous episode of allergy to polysulfone during HD at the hospital.
The patient remained on chronic HD for 9 months. Over that period of time, she developed residual diuresis (approximately 500 mL/day) and stabilization of sCr levels around 4.3 mg/dL, with the possibility for HD suspension. At this time, renal scintigraphy was performed and revealed a left kidney with heterogeneous uptake (87%), more reduced in the lower half, suggesting the presence of cortical cicatricial lesions. The right kidney presented severely reduced uptake (13%), probably related to extensive cortical cicatricial lesions. The renogram showed severely reduced estimated renal tubular function ensured almost exclusively by the left kidney (96%). The patient is currently being followed in Nephrology Outpatient Clinic, with baseline sCr of 3.5 mg/dL.
DISCUSSION
We present a rare case of bilateral renal artery thrombosis that occurred in two different times: in the first instance, thrombosis of the right renal artery occurred, with progressive homolateral kidney atrophy and dysfunction and, three months later, of the left artery, leading to severe renal disease with oliguria and need for renal replacement therapy. Saju et al presented a review on renal infarction, and the incidence described was 0.004% (20 of 481 540), with the most common etiology being cardiac origin (75%), and right‑sided renal infarctions were more common than a left‑sided and bilateral disease.4 This patient presented no history of cardiovascular disease nor recent trauma. The cause of bilateral thrombosis of the renal arteries was not identified, with cardioembolic conditions and hypercoagulability states being excluded (Table 1). However, some risk factors could be identified, such as the past history of smoking4 and SARS‑CoV‑2 infection five months before. Thromboembolic events are a serious consequence of coronavirus disease (COVID‑19), with several cases of renal artery thrombosis related to the infection having been reported, including as a long‑term complication in rare cases, which could have occurred with our patient5,6; this is mainly related to the direct effect of SARS‑CoV‑2 on the endothelial cells with consequent hyperinflammatory response and cytokine storm, complement activation, mononuclear phagocytes, neutrophil extracellular trap formation, tissue hypoxia and, finally, thrombosis.7
Clinical presentation was very unspecific, as described in most studies, which raises awareness for the importance of establishing renal artery thrombosis as a differential diagnosis in the setting of acute abdominal or flank pain, nausea, vomiting, fever and hematuria.2,8Due to this frequent unspecific presentation, diagnosis of renal infarction is delayed or can be missed, which may lead to irreversible loss of renal function, as renal reperfusion therapy is ineffective when prolonged damage is installed.1,2
The initial investigation in patients presenting with acute flank pain, vomiting, and hematuria is usually an ultrasound or a non‑contrast CT scan of the abdomen and pelvis to rule out pyelonephritis and urolithiasis.4 However, some authors defend that when a triad of persistent flank/ lower back pain, high lactate dehydrogenase (LDH) levels and/or hematuria are present, a contrast‑enhanced CT should be performed within 24 hours of the clinical presentation.9 Renal CT arteriography is the gold standard exam for diagnosis of renal infarction. Other exams are magnetic resonance imaging (MRI) with gadolinium contrast excretion and renal MRI arteriography. A color Doppler scan demonstrates decreased or absent blood flow to the infarcted area.4,6Regarding laboratory findings, a strong correlation with hematuria, proteinuria, elevated aspartate aminotransferase (AST), and elevated LDH has been noted once the thrombosis has progressed to renal infarction.1,3
After the diagnosis, particularly in atraumatic situations, an electrocardiogram should be performed to exclude atrial fibrillation, as well as an echocardiogram to evaluate for cardioembolic origin, especially if cardiac valvular prosthesis and history of atrial fibrillation are present.4 It is crucial to investigate the use of medications and substances associated with higher thrombotic risk, the presence of systemic disorders, in particular connective tissue conditions, and hypercoagulability disorders, such as hereditary or acquired thrombophilia, including anti‑phospholipid syndrome.6
Given the rarity of this disease, guidelines on therapeutic approach are not well standardized.10 A recent review study of Saju and Leslie presented a summary of the available treatment options.4 CT renal angiography allows transcatheter treatment for acute cases, when appropriate; thrombectomy is usually tried first, and then thrombolysis, with suggested optimal door‑to‑treatment time being 90‑180 minutes.11 Thrombolytics continuous infusion can be considered for up to 72 hours if bolus treatment is unsuccessful. Angioplasty and/or arterial stenting may be used when thrombolysis fails or is inappropriate. Open surgery is appropriate for trauma cases and those associated with aortic dissections.10 Systemic anticoagulation is recommended for late diagnosed cases and is usually given for at least six months.4
Even though it is known that renal infarction is settled after 8 hours of ischemia, successful revascularization has been described after this period,12 as renal collateral vascular supply seems to partially sustain renal viability and the potential for the kidney to regenerate after acute tubular lesion.13 However, determining the onset of the infarction may be extremely difficult,4 which influences inevitably the treatment approach.
Medical therapy with antiplatelet drugs, antihypertensive agents and statins are crucial for the management of patients with cardiovascular risk factors, and when atherosclerotic renal artery disease is suspected or documented.14 However, when full‑dose anticoagulation is indicated, the addition of antiplatelet therapy should generally be avoided because of bleeding risk, unless a recent percutaneous revascularization was performed.6,14Nevertheless, guidelines are scarce regarding medical management of renal artery thrombosis. Most antihypertensive drugs are effective for treating hypertension in these patients. Angiotensin‑converting enzyme inhibitors (ACEIs) and angiotensin‑receptor blockers (ARBs) may be introduced in the case of bilateral RAS and when the lesion affects a single functioning kidney, provided that the patients are very carefully monitored, since in large observational studies they have shown benefits in reducing mortality and morbidity in patients with renal artery disease, despite having no impact in thrombotic events.14 There are no recommendations regarding the monitoring and follow‑up after diagnosis of renal artery thrombosis, but considering the potential for renal recovery, particularly in patients with need for renal replacement therapy, monitoring should be frequent.
The prognosis is assessed by the size of the thrombosis, duration, and scope of the infarction. Acute kidney injury is frequent when pre‑existing renal disease occurs, even in cases of segmental arterial occlusion.4 In case of renal infarction, 8% of patients will need dialysis, and most will have improved renal function once perfusion of the kidney is reestablished.3 The degree of arterial blockage can be determined by CT renal angiography, and partial occlusion with some degree of parenchymal perfusion suggests potential recovery, as occurred in our patient. Typically, even complete occlusions of 6 hours are considered potentially reversible.4
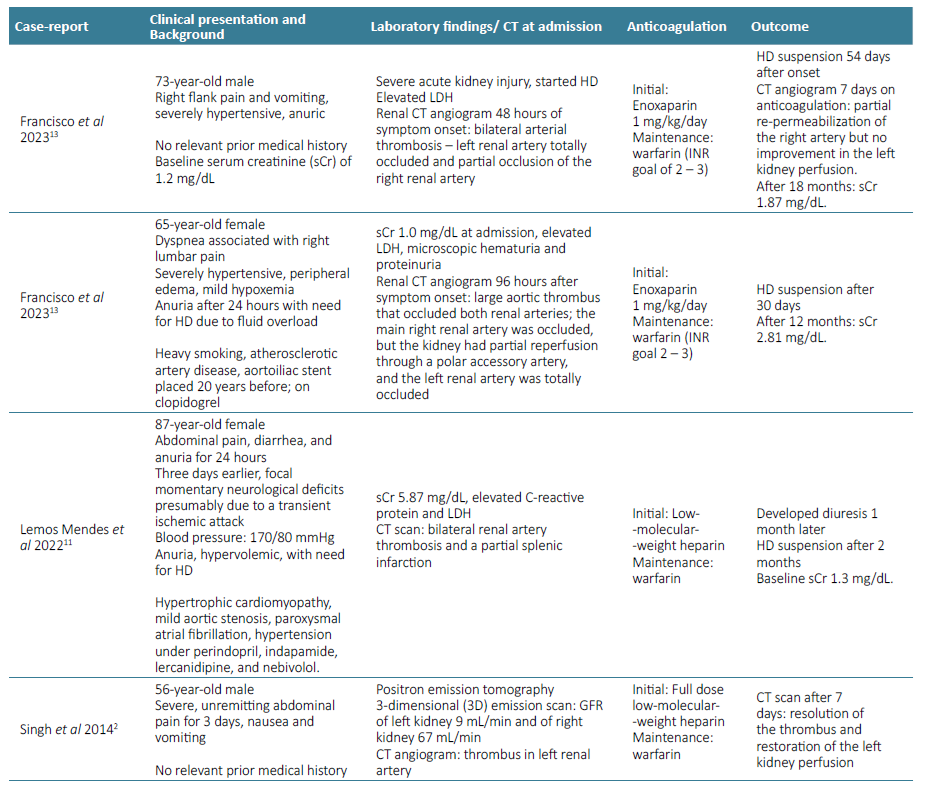
There are few cases of bilateral renal artery thrombosis with renal recovery treated only with anticoagulation monotherapy described in literature (Table 2). All of them used warfarin as the maintenance anticoagulation of choice. Francisco et al presented one case of bilateral renal artery thrombosis, documented 48 hours after symptom onset, which lead to anuric AKI. The patient became HD‑dependent and was discharged with maintenance warfarin treatment. Progressive recovery of kidney function was observed, and, after 54 days, HD was suspended.13 Also, Lemos Mendes et al published a case of an 87‑year‑old woman with bilateral renal artery thrombosis, admitted with abdominal pain, diarrhea, and anuria, for the last 24 hours. Lab work at admission showed sCr 5.9 mg/dL, urea 100 mg/dL, and HD was initiated due to fluid overload. Anticoagulation was started, first with low‑molecular‑weight heparin, and then warfarin. One month later, the patient developed diuresis and, two months later, she no longer required dialysis, with nadir sCr of 1.3 mg/dL.11 After nine months of anticoagulation with apixaban, our patient presented progressive improvement of diuresis and HD was suspended. To our knowledge, this is the first case of renal artery thrombosis, treated with apixaban on monotherapy, presenting with renal function recovery. The safety profile and a more suitable administration made apixaban the choice for our patient. More studies are needed to define the most safe and effective oral anti‑coagulant in these cases, as well as the timing to initiate it and for how long it should be maintained. 4
CONCLUSION
We present a case of late diagnosed renal artery thrombosis that ended up with the need for renal replacement therapy. Clinical presentation with flank pain should raise suspicion for this usually overlooked condition, as prompt diagnosis and revascularization treatment are crucial to improve renal outcomes. Despite the delay in diagnosis and severity of the kidney injury, the patient presented a favorable outcome with the possibility for HD suspension, after 9 months of oral anticoagulation with apixaban. To our knowledge, this is the first case of renal artery thrombosis, treated with apixaban on monotherapy, with renal function recovery.