INTRODUCTION
Chronic kidney disease (CKD) is a complex and heterogeneous disease, that poses an increasingly global public health concern, affecting approximately 11% to 13% of the world’s population.1‑3Regardless of the underlying etiology, CKD is simultaneously characterized by persistent proinflammatory4 and immunosuppressive states due to the impact of the uremic milieu.5 Peritoneal dialysis (PD), which accounts for 9% of all kidney replacement therapy (KRT) and 11% of all dialysis modalities,6 may have an additional inflammatory factor related to chronic exposure to high‑load glucose solutions.
Over time, some changes are expected to occur in the peritoneal membrane (PM) primarily due to exposure to PD solutions, infectious processes (particularly PD‑associated peritonitis) and non‑infectious complications (such as catheter dysfunction).7,8Currently, biocompatibility of PD solutions may represent the main issue affecting the durability of PD. Chronic exposure to PD solutions, which are non‑physiological, traditionally containing an acidic pH and high concentrations of glucose,9 will eventually lead to deleterious processes that affect the function and morphology of the peritoneum. Another significant problem that can compromise PD function and maintenance of the technique is infectious peritonitis.10 Overall, PM failure results from cellular and molecular changes,11 characterized by increased production of proinflammatory mediators, proangiogenic and profibrotic factors,12,13which respectively lead to angiogenesis and fibrosis.14 Among these inflammatory mediators, interleukin 17‑A (IL‑17A), as an effector cytokine, and its main producer, T helper 17 (Th17) cells, seem to stand out. In recent years, evidence from animal studies and a few human studies have demonstrated IL‑17A‑mediated PM effects, including local inflammation and progressive peritoneal damage.15‑17Thereby, IL‑17A is thought to stimulate the development of fibrosis and angiogenesis that compromise the adequate function of the PM,18,19although other reports may suggest otherwise.20 The imbalance of immune cells, namely Th1 and Th2 cells, is typically characterized by a decreased Th1/ Th2 ratio in peripheral blood.21 The shift of TCD4+ cells towards Th2 cells has been implicated in the increased risk of thickening of the sub mesothelial space and PM fibrosis.22 The classical role of immune cells in the setting of PD, and more specifically in the peritoneal effluent (PE), may thus be challenged by exposure to PD solutions, with unphysiological glucose concentrations, recurrent peritonitis or contact with synthetic materials like the PD catheter.
To better elucidate the immune changes associated with long‑term PD, and because factors other than those related to the glucose solutions can have important effects on the PM,9 we aimed to characterize proinflammatory immune cell subsets (mainly Th1 and Th17 cells), as well as IL‑17A levels in blood and PE from a subset of PD patients, looking at PM and PD prescription characteristics as potential modulators of blood and PE immune cell profile.
MATERIAL AND METHODS
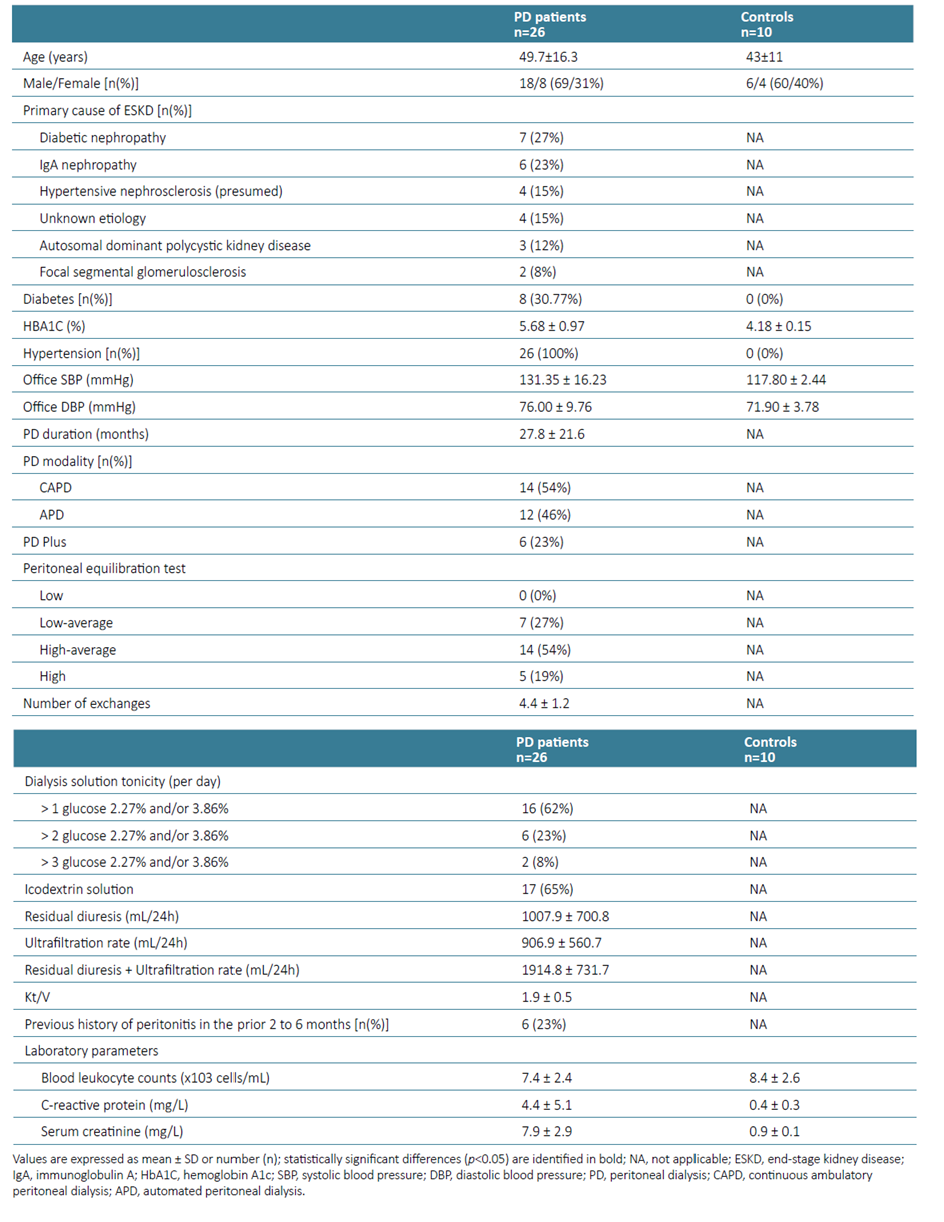
Twenty‑six chronic PD patients aged≥ 18 years and 10 healthy controls were recruited from routine consultations. All participants provided written informed consent and approval from the Ethics Committee of ULS Castelo Branco, EPE‑Hospital Amato Lusitano was obtained (IRB approval number: 014862). None of the patients was on glucocorticoids or another immunosuppressive agent in the last 6 months or had a diagnosis of peritonitis or another active infectious disease in the last 30 days. Ten healthy, age and sex‑matched volunteers were included as the control group. They had no medical history of hypertension, diabetes, or immune‑related disorders, as well as any ongoing inflammatory process, as accessed by the measurement of serum C‑reactive protein levels (CRP). PD patients were subsequently evaluated according to dialysis modality [continuous ambulatory peritoneal dialysis (CAPD) versus automated peritoneal dialysis (APD)], glucose exposure [≥ 3 hypertonic (defined as glucose 2.27% and/or 3.86%) solutions/day,)], icodextrin use, dialysis efficiency (urea Kt/V) and previous history of peritonitis in the prior 2 to 6 months.
Data about residual diuresis and peritoneal solute transport rate (PSTR) were also obtained. The PSTR was determined by the fast peritoneal equilibration test (PET) based on the 4‑hour dialysate/plasma (D/P) creatinine (Cr) and recorded as a continuous variable.23 Drain volume over the 4‑hour was recorded as the UF volume.
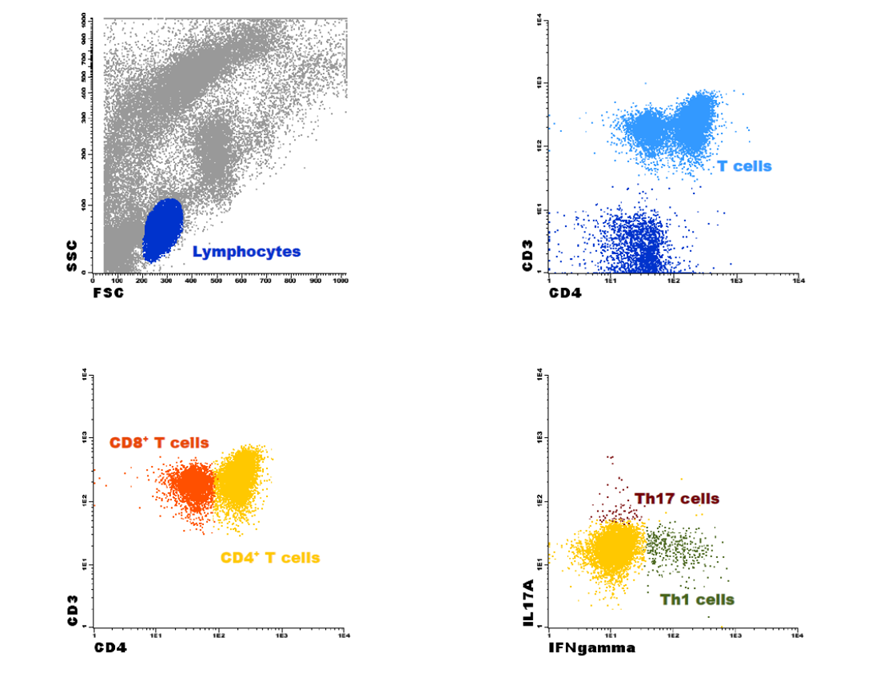
For PD patients, blood and PE samples were simultaneously collected, avoiding a time lag between samplings. Peripheral blood collection was done for a heparin tube for the flow cytometry analysis, and in a serum‑gel tube for the ELISA assay. The latter was centrifugated at 3000 rpm for 10 min and the serum was frozen at‑20ºC. PE samples were collected in sterilized containers and all samples were analyzed within 24 hours. To maximize immune cell identification, centrifugation at 2000 rpm for 5 minutes before the following protocol was performed. The collection of PE did not apply to control patients. Absolute peripheral blood and PE cell counts were calculated using a Yumizen H2500TM (Horiba, Kyoto, Japan) hematological cell analyzer. To measure IL‑17A levels, the samples were activated with a cocktail containing brefeldin A, phorbol 12‑myristate‑13‑acetate (PMA) and ionomycin. Peripheral blood and PE samples were diluted in RPMI‑1640 medium, in the presence of the referred cocktail and incubated at 37ºC in a sterile environment with a 5% CO2 humid atmosphere for 4 hours. After activation, samples were aliquoted in two tubes (one peripheral blood sample/tube and one PE sample/tube), one of them being the control (without antibodies). For staining intracellular cytokines, Cytofix/CytopermTM Fixation/Permeabilization Kit, a permeabilization and fixation protocol was followed. According to the manufacturer’s instructions, the samples were initially stained for the surface antigens with mouse anti‑human monoclonal antibodies (anti‑CD3 and anti‑CD4 for peripheral blood samples and anti‑CD3 and anti‑CD4 for PE samples), incubated for 15 minutes in the dark at room temperature (RT), washed with phosphate‑buffered saline (PBS) and centrifuged at 2000 rpm for 5 minutes. The cell pellet was then resuspended in fixation/ permeabilization solution, incubated for 20 minutes in the dark at RT and centrifuged at 2000 rpm for 5 minutes. A washing step was performed next with BD Perm/WashTM buffer (diluted 1:10 in distilled H2O), followed by a 10‑minute incubation period in the dark at RT and centrifuged at 2000 rpm for 5 minutes. For the intracellular staining, anti‑IFN‑γ, anti‑IL‑17A (peripheral blood samples), anti‑IFN‑γ and anti‑IL‑17A (PE samples) were added and incubated for 30 min in the dark at RT. Another washing step was performed, after which the cell pellet was resuspended in PBS and samples were immediately acquired in a FACSCaliburTM (BD Biosciences, San Jose, CA, USA) flow cytometer. For each sample and whenever possible, 1x104 events were acquired and analyzed using InfinicytTM software (version 1.8, Cytognos SL, Salamanca, Spain). To quantify the T cell subsets, total lymphocytes population was identified based on forward (FSC) and side (SSC) scatter properties. Within this cell population, T cells were identified based on the expression of CD3 and subsequently the CD4+ T cells, according to the ex‑ pression of CD4+. CD8+ T cells were identified by exclusion, as CD4‑ CD3+ T cells. Considering the expression of IL‑17A and IFN‑γ, Th17 and Th1 cells were identified, respectively, as CD4+ IL‑17A+ IFN‑γ‑ and CD4+ IL‑17A‑ IFN‑γ+.24 Fig. 1 demonstrates the flow cytometry gating strategy. (Additional data about the reagents and antibodies in Supplementary Material). To measure the expression levels of IL‑17A in the serum and effluent samples, LEGEND MAXTM Human IL‑17A ELISA Kit, an enzyme‑linked immunosorbent assay (ELISA) was used following manufacturer’s instructions (Supplementary Material).
Data are presented as mean ± standard deviation (SD). The Shapiro‑Wilk test was applied to check for normality. Univariate analysis was applied to search for differences between blood and PE as well as for differences in blood from PD patients and controls. Whenever normal distribution was documented, independent samples t‑test or one‑way ANOVA were applied. Non‑parametric tests (Mann‑Whitney or Kruskal Wallis) were used for variables with non‑normal distribution. For comparisons including three or more groups, post‑hoc analyses were performed. Pairwise comparisons were also done accordingly, using the T‑test or Wilcoxon test as appropriate. SPSS statistics version 26 was used and a p‑value of p<0.050 was considered statistically significant.
RESULTS
Demographic and baseline characteristics
The baseline characteristics of PD patients (n=26) and healthy control group (n=10) are shown in Table 1. The mean age of the PD patients was 49.7 ± 16.3 years, 69% were male, eight (30.77%) were diabetic and all of them had a previous history of hypertension. There were no significant differences in terms of gender and age between the PD patients and controls. The mean time on PD was 27.8 ± 21.6 months and 14 (54%) were on CAPD. Six patients (23%) had a previous history of peritonitis in the prior 2 to 6 months.
Peripheral blood T cell subsets in PD patients and healthy controls
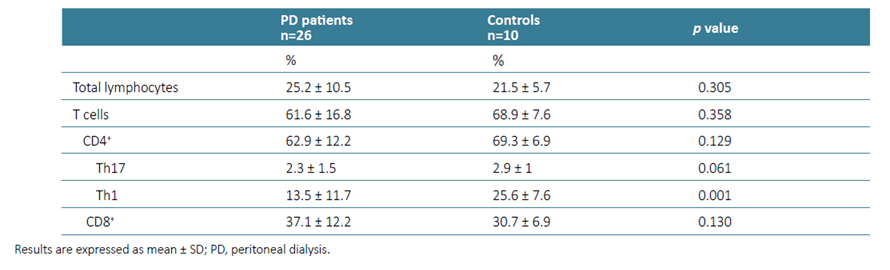
Among the major subsets of T cells, no differences were observed between controls and PD patients, with the per‑ centage of TCD4+ and TCD8+ cells being identical between the two groups (p=0.129, p=0.130, respectively). However, the frequency of Th1 cells was significantly decreased in PD patients’ blood (p=0.001). A lower percentage of Th17 cells was also observed, although not statistically significant (p=0.061), (Table 2).
Peripheral blood and peritoneal effluent T cell subsets in PD patients
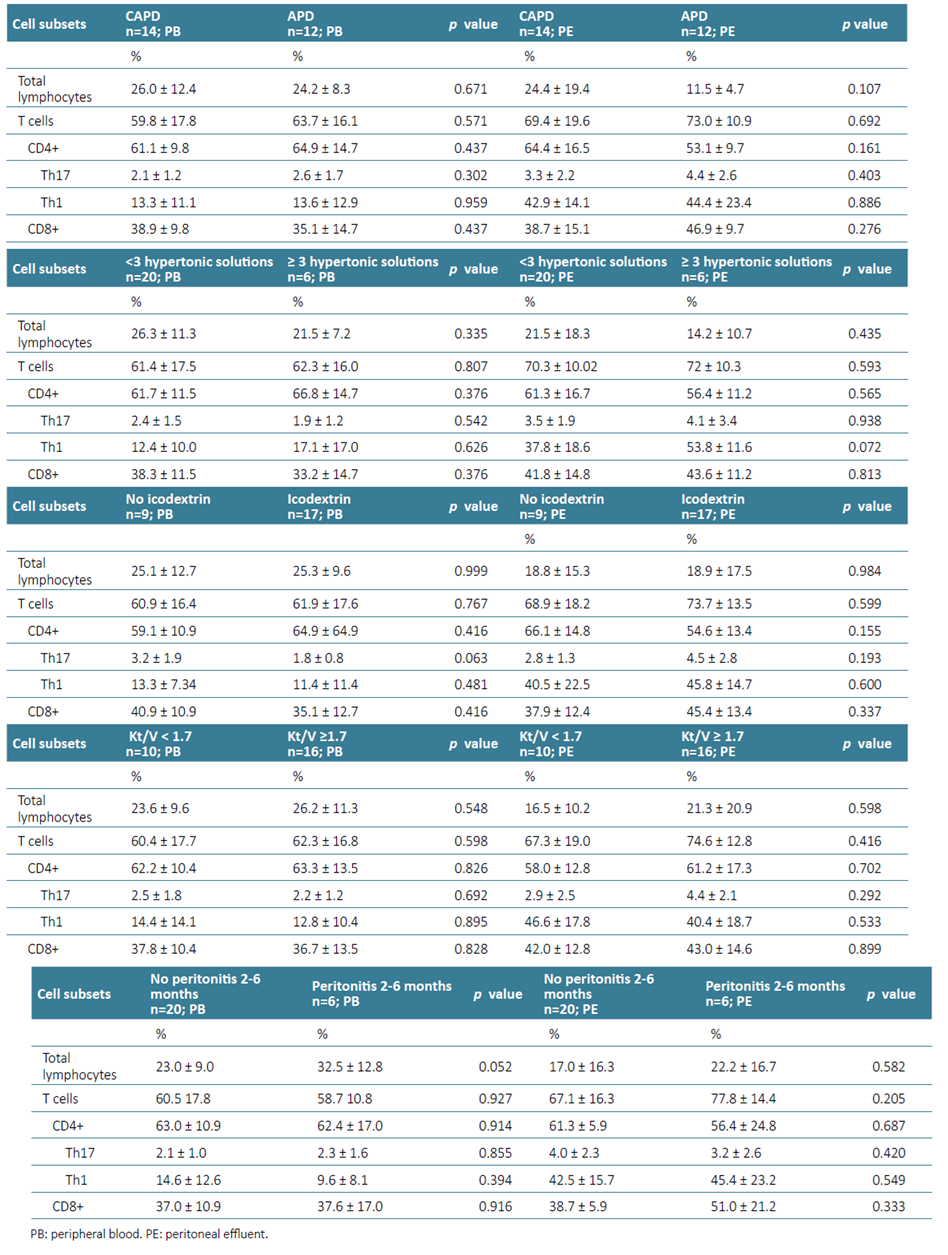
Data from blood and PE from PD patients, based on dialysis technique (CAPD versus APD), daily glucose loading (≥ 3 hypertonic solutions/day), icodextrin use, dialysis efficiency as determined by urea Kt/V ≥1.7, history of peritonitis in the previous 2 to 6 months are described in Table 3 and PSTR in the Supplementary Material.
Dialysis modality
The percentages of total lymphocytes, T cells and T cells subsets (TCD4+, Th17, Th1 and TCD8+) were identical between the different PD modalities (Table 3). In our sample, the average number of exchanges/day in APD patients was 5.5 ± 0.7 and in CAPD patients was 3.4 ± 0.5.
Daily glucose loading
Daily glucose loading was evaluated based on the number of hypertonic solutions used, and a cut‑off of ≥ 3 hypertonic solutions/day was defined as a high glucose exposure measure. Only 6 patients fulfilled this criterion. In this setting, no differences were found that could contribute to a different immune cell profile in peripheral blood. However, patients with 3 or more hypertonic solutions/day showed a trend towards higher frequency of Th1 cells (p=0.072) in PE.
Icodextrin use
A high proportion of the patients in our cohort had a prescription of icodextrin, which appears to contribute to a further reduction in the percentage of peripheral Th17 cells (p=0.063). When compared to the healthy cohort (Supplementary Material), these reductions were even more relevant (p=0.030). These reductions did not result in an overall decrease in T or TCD4+ cells. No significant differences were observed in the PE according to icodextrin use, with the percentage of immune cells identified being rather similar between APD and CAPD patients.
Dialysis efficacy
No differences were documented in the peripheral blood and PE immune cell profile in patients with adequate or low dialysis efficacy. The recommended cut‑off of Kt/V was applied, but in this cohort, it did not contribute to an increase in proinflammatory cells (p>0.05) in either of the PD modalities considered.
Previous history of peritonitis
As previously mentioned, patients with an ongoing or recent (<1 month) episode of peritonitis were not included in this analysis. Patients with a previous history of peritonitis occurring within the prior 2 to 6 months did not exhibit statistically significant differences in peripheral blood and PE immune cell profiles.
Peritoneal solute transport rate
The frequency of total lymphocytes was increased in the PE of high transporters when compared to high‑average transporters (p=0.021) and compared to low‑average transporters, although this result did not reach statistical significance (p=0.078). No other differences were found according to the type of PM transport (Supplementary Material).
Peripheral blood and peritoneal effluent immune cell profiles
The comparison of blood and PE shows that the frequency of Th1 cells was significantly higher in PE (p<0.001) (Fig. 2). The frequencies of the remaining immune cells were similar in blood and PE, with PE closely resembling peripheral blood numbers (Supplementary Material).
IL‑17A in peripheral blood and peritoneal effluent
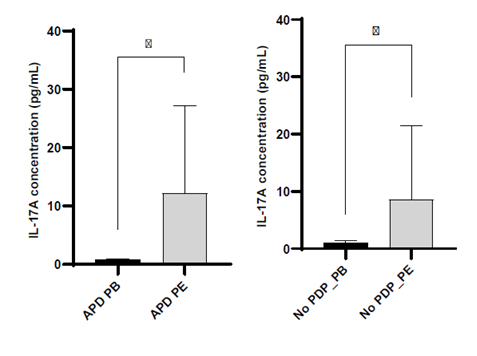
Blood levels of IL‑17A were similar between PD patients and controls (Supplementary Material). Although a trend towards a reduced percentage of Th17 cells was observed in the blood of PD patients, this finding did not translate into differences in serum IL‑17A concentrations. However, IL‑17A levels were significantly increased in the PE (p=0.039) (Fig. 2), despite a similar percentage of Th17 cells in blood and PE. This pattern of increased IL‑17A in PE was independent of daily glucose exposure, the use of icodextrin, previous history of peritonitis and PSTR. How‑ ever, for patients on APD, increased levels of IL‑17A were seen in the PE (Fig. 3).
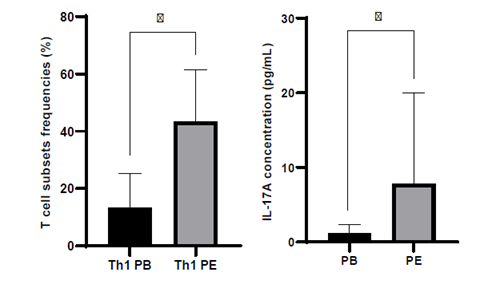
Figure 2. Analysis of immune cell frequencies and IL‑17A levels in blood and peritoneal effluent from peritoneal dialysis patients.
DISCUSSION
This study aimed to characterize proinflammatory immune cell subsets (mainly Th1 and Th17 cells), as well as IL‑17A levels in blood and PE, looking at PM and PD prescription characteristics as potential modulators of blood and PE immune cell profile of a subset of PD patients. Focusing on the analysis of peripheral blood, the expected increase proinflammatory (Th1 and Th17) cells in the peripheral blood of PD patients was not documented in our cohort. Even if high glucose exposure may render these patients more prone to cardiovascular disease and its complications,16 because of volume overload and advanced glycosylation end product deposition, this was not reflected in a more aggressive proinflammatory immune cell profile. We may hypothesize that Th1 and Th17 cells may not be the link between PD and high cardiovascular risk or, alternatively, that a parallel expansion of regulatory T cells may occur to overcome inflammation, as observed in other chronic conditions.25 Regulatory T cells and their associated cytokines should be evaluated in future investigations to draw further conclusions. We also documented a lower frequency of Th1 cells in peripheral blood. Although we have not quantified the frequency of Th2 cells, our results seem to be in accordance with previous studies that demonstrated a decrease in Th1/Th2 ratio in PD patients.21 Concerning Th17 cells, our results differ from previously published data since most authors report an increase in this subset of cells.26,27However, only one of those reports26 considers patients undergoing PD, with no information available regarding the characteristics of the treatment.
Given the limited research made outside animal models and the scarce human data, a more in‑depth analysis urges to understand the biology of immune cells in such peripheral blood and PE dynamics.
Th17 cells, and its main secreted cytokine, IL‑17A, have been in the spotlight as relevant mediators in the pathogenesis of several chronic inflammatory disorders, including renal diseases.28‑30Surprisingly, the first evidence of the potential role played by IL‑17A in renal inflammation was reported before the functional significance of Th17 cells was discovered.31 Experimental data suggests that both seem to be involved in the cumulative damage and deterioration observed during long‑term PD treatment.15,32It is also important to be aware that other sources of IL‑17A, rather than Th17 cells, can release this cytokine to the extracellular milieu, after pathological reshaping, contributing to increased levels that are not only explained by the observed Th17 cells frequency. In our cohort, IL‑17A concentration was higher in PE without a concomitant increase in Th17 cells.
According to PD prescription, it is noteworthy that the type of PD modality did not contribute to differences in the immune cell profile between peripheral blood and PE. To our knowledge, no published studies so far have specifically emphasized the comparison of the immunological profile in blood and PE between the two modalities. CAPD and APD demonstrated absence of statistically significant differences in the several parameters studied. Thus, PD modality had no impact on the overall immune profile in our cohort.
Exposure to a high glucose overload or use of icodextrin-based solutions may induce some changes in the immune cell profile that, in the long term, may be relevant for peritoneum preservation and PD survival. Prescription of solutions with high glucose concentration appears to have contributed to the increased frequency of Th1 cells in the peritoneal cavity, while the use of icodextrin‑based solutions promoted a further downregulation of Th17 cells in the blood, although both results did not reach statistical significance, probably because of the small sample size (p=0.072; p=0.063, respectively). These findings led us to hypothesize about an immuno‑ modulatory action of glucose and icodextrin on the PM. Additionally, icodextrin treated patients had a lower frequency of Th17 cells compared to healthy controls, supporting this hypothesis. The benefits of icodextrin are well documented, allowing better management of hypervolemia and hypertension, especially in patients with defective ultrafiltration.33 It also promotes reduced peritoneal glucose exposure and absorption, which may prolong patient and PD technique survival as well as improve metabolic control in diabetics.34 Furthermore, the concept that Th17 cells play a pathogenic role in hypertension has been widely accepted35,36and, therefore, strategies to manage the population of Th17 cells are welcome. Exploring a potential immunoregulatory role for icodextrin in PD patients, along with studying other immune cell subsets, pursued to develop strategies that can modulate the increased cardiovascular risk associated with long‑term PD.
Regarding the characteristics of the PM, there were no differences documented in the peripheral blood and PE immune cell profile in patients with adequate or low dialysis efficacy. The recommended target of kt/v did not influence the immunological profile of PE or peripheral blood, which may be unbalanced in conditions of chronic uremia.5 Markers of residual inflammation secondary to a previous history of peritonitis were also not documented in our analysis, both in peripheral blood and PE, confirming complete resolution of the infectious process and absence of ongoing inflammatory cells proliferation. Interestingly, the frequency of total lymphocytes was increased in PE of high transporters compared to high‑average transporters, suggesting increased membrane permeability as a facilitator of leukocytes translocation. The difference between high transporters and low‑average transporters did not reach statistical significance, probably due to the small sample size, and should be reassessed in a larger study in the future.
Unexpectedly, a significant similarity was observed between the immune cell profiles in peripheral blood and PE. The distribution of immune cells in PE closely resembled that of peripheral blood, except for Th1 cells. We speculate that the high glucose gradient between blood and peritoneum may serve as an additional stimulus for the migration of Th1 cells, leading to an immune‑mediated inflammatory process predominantly driven by Th1 cytotoxicity. Recent data has shown that, regardless of PD solution used in CAPD patients, the number of Th1 IFN‑γ‑producing cells in peripheral blood is significantly reduced compared to controls, while significantly increased in the PE (when cells were stimulated, compared to their unstimulated counterparts).37 Such Th1‑like immune response in the PE has been linked to a lower risk of peritonitis, as these cells produce high levels of IFN‑γ, enhancing the killing capacity of peritoneal cells.38,39
Our results also challenge the concept of a nearly sterile peritoneal cavity in non‑pathological conditions, by identifying several proinflammatory immune cells, as well as increased levels of pathological interleukins, such as IL‑17A, in a peritonitis‑free situation. Overall, in PD patients chronically exposed to high glucose concentration solutions, increased migration or local proliferation under the glucose stimulus may explain these differences. Increased IL‑17A in PE also contributes to an increased proinflammatory burden on the PM.
Several limitations need to be addressed in our study, including not only the small sample size but also the lack of a completely standardized technique for measuring and quantifying immune cells and IL‑17A levels in PE. For instance, since IL‑17A levels can be affected by ultrafiltration, which in turn can be influenced by PSTR and dwell time, the determination of the appearance rate of IL‑17A levels could be a more suitable approach. Future research should also address a broader spectrum of immune cells and other proinflammatory factors. It would be relevant to investigate how Th1 cells relate to Th2 cells (Th1/Th2 ratio). Likewise, the balance between Treg and Th17 cells seems to be of interest since the predominance of Th17 cells over Treg is said to lead to fibrosis development and peritoneal damage.40 One of the most valuable strengths of our work is the fact that we were able to analyze and compare both peripheral blood and PE samples from PD patients. This allowed us to better characterize the immune profile of these patients, incorporating specific details of the dialysis prescription and PM characteristics.
CONCLUSION
Long‑term PD seems to modify both circulating and peritoneal immune cell profiles. To our knowledge, this is one of the few human studies that addressed the differential distribution of pro‑inflammatory immune cells in peripheral blood and PE of PD patients. PM characteristics and PD prescription such as dialysis modality, daily glucose exposure, PD efficiency and history of peritonitis (2 to 6 months prior) did not influence peripheral immune cell profiles. In our cohort, we found an increase in local inflammatory response in the PE, as evidenced by elevated concentrations of IL‑17A, rather than an increase in the systemic inflammatory response. This increase was not accompanied by a rise in Th17 cells, suggesting an alternative source of IL‑17A production. Human studies with larger sample sizes are needed to draw definite causal conclusions.