INTRODUCTION
Chronic kidney disease (CKD) is a contemporary public health issue, with an estimated worldwide prevalence ranging from 7% to 15%.1‑3The growing incidence of cardiovascular and renal risk factors, such as hypertension, diabetes and obesity, associated with improved medical care, have allowed more people to live long enough to develop CKD with the need for kidney replacement therapy.4According to the United States Renal Data System (USRDS), in 2019, 134 608 individuals were newly diagnosed with end‑stage renal disease (ESRD), with an adjusted incidence of 386 cases per million population.5 The prognostic impact of ESRD is also noteworthy, as it is associated with an increased number of hospitalizations, healthcare costs and mortality.6
Patients with progressive decline in kidney function should be referred for dialysis access assessment and subsequent creation when eGFR is 15‑20 mL/min/1.73 m2. Earlier referral should also occur in patients who are expected to go on dialysis in less than 6 months.7,8
An arteriovenous fistula (AVF) or an arteriovenous graft (AVG) are preferable to a central venous catheter (CVC). Using a CVC for HD is associated with a much higher risk of vascular access‑related events such as infection, thrombotic and nonthrombotic complications, bacteremia, infection related hospitalizations and mortality compared to those who achieve an AVF or AVG as HD access.7‑9 Therefore, several international clinical practice guidelines (CPG) recommend a “fistula first” approach based on the best long‑term outcomes, lowest mortality, and lowest health care costs of AVF compared with AVG or CVC.10‑13 However, in the United States, still over 80% of patients start HD with a CVC14,15 The choice of vascular access (VA) should also take into consideration the patient’s life expectancy, degree of dependence and personal preferences, particularly when referring to patients with complex medical comorbidities and geriatric syndromes.16 The increase of elderly patients on HD represents a challenge for access planning as these patients often have worse vascular conditions associated with an increasing prevalence of diabetes, coronary and peripheral artery disease. These cardiovascular comorbidities increase the chance of primary failure and need for assisted maturation which negatively impacts the mortality associated with renal replacement therapy.17
As a population with special characteristics, it would be expected that elderly patients would have specific indications referring to vascular access. However, CPGs address this issue sparsely, as there is no reference on how to manage this specific population.17‑19
Our study aimed to evaluate if the initial vascular access defined patient prognosis and whether the transition from a CVC to an AVF or AVG improved very elderly patient survival.
MATERIAL AND METHODS
The authors performed a retrospective analysis of adult patients who initiated HD between January of 2014 and December of 2019 in Centro Hospitalar Universitário Lisboa Norte (CHULN) in Lisbon, Portugal. The Ethical Committee approved this study, in agreement with institutional guidelines. Informed consent was waived, given the retrospective and non‑interventional nature of the study.
Participants
We selected as eligible all patients aged ≥ 80 years old who initiated HD from January 1st of 2014 to December 31st of 2019 and who remained dialysis dependent at the time of discharge. We considered both urgent and planned RRT start. We excluded patients who died in the first 90 days after dialysis start. Patients with previous RRT, namely peritoneal dialysis or renal transplant, were excluded, as were patients lost to follow‑up.
Variables and outcomes
Data was obtained from individual electronic clinical records (EHR). The following variables were collected: demographic characteristics (age, gender, race); comorbidities [CKD, hypertension, diabetes mellitus, heart failure, ischemic cardiomyopathy, cerebrovascular disease, peripheral artery disease, rheumatic disease, chronic hepatic disease, chronic obstructive pulmonary disease (COPD), active malignancy, dementia]; HD access at the time of HD start (CVC, AVF, AVG); laboratory at HD start [hemoglobin, neutrophil and lymphocyte count, platelet count, serum urea, serum creatinine, estimated glomerular filtration rate (eGFR), serum albumin, serum ferritin, serum parathyroid hormone (PTH), and C‑reactive protein (CRP).
The primary outcome was mortality within one year of HD start. Secondary outcomes were primary failure of vascular access in those who started HD with a CVC and three‑year mortality.
Definitions
The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‑EPI) creatinine equation. The presence of CKD was defined as an eGFR lower than 60 mL/min/1.73 m2 known for at least 3 months before presentation. Hypertension was diagnosed according to European Society of Cardiology and European Society of Hypertension Guidelines. Diabetes mellitus was defined in accordance with American Diabetes Association Guidelines. Heart Failure was considered based on previously known clinical diagnosis of any cause. Ischemic cardiomyopathy included both previous myocardial infarction and chronic coronary artery disease and was based on a prior known diagnosis. Cerebrovascular disease was defined based on a prior history of stroke, carotid, vertebral or intracranial stenosis, aneurysms or vascular malformations. Peripheral arterial disease and dementia were considered based on previously documented clinical diagnosis. Rheumatic disease included all previously diagnosed autoimmune and inflammatory diseases. Chronic liver disease was defined as a deterioration of liver function for more than six months of all causes, as previously documented on the clinical history. COPD included emphysema and chronic bronchitis.
Statistical methods
Categorical variables were described as the total number and percentage of each category, while continuous variables were described as the mean ± standard deviation. The Kolmogorov‑Smirnov normality test was used to examine if variables were normally distributed. Continuous variables were compared using Student’s t‑test, whereas categorical variables were compared using the Chi‑square test. All variables were submitted to univariate analysis to find statistically significant factors that could be predictive of mortality within the first year of HD start. Subsequently, variables with a significant statistical difference underwent multivariate analysis using the Cox logistic regression method. Data were conveyed as odds ratios (OR) with 95% confidence intervals (CI). Median overall survival was analyzed using Kaplan‑Meier curves and the log‑rank test. Statistical significance was established as a p‑value lower than 0.05. Statistical analysis was achieved using the statistical software package SPSS for Windows (version 21.0).
RESULTS
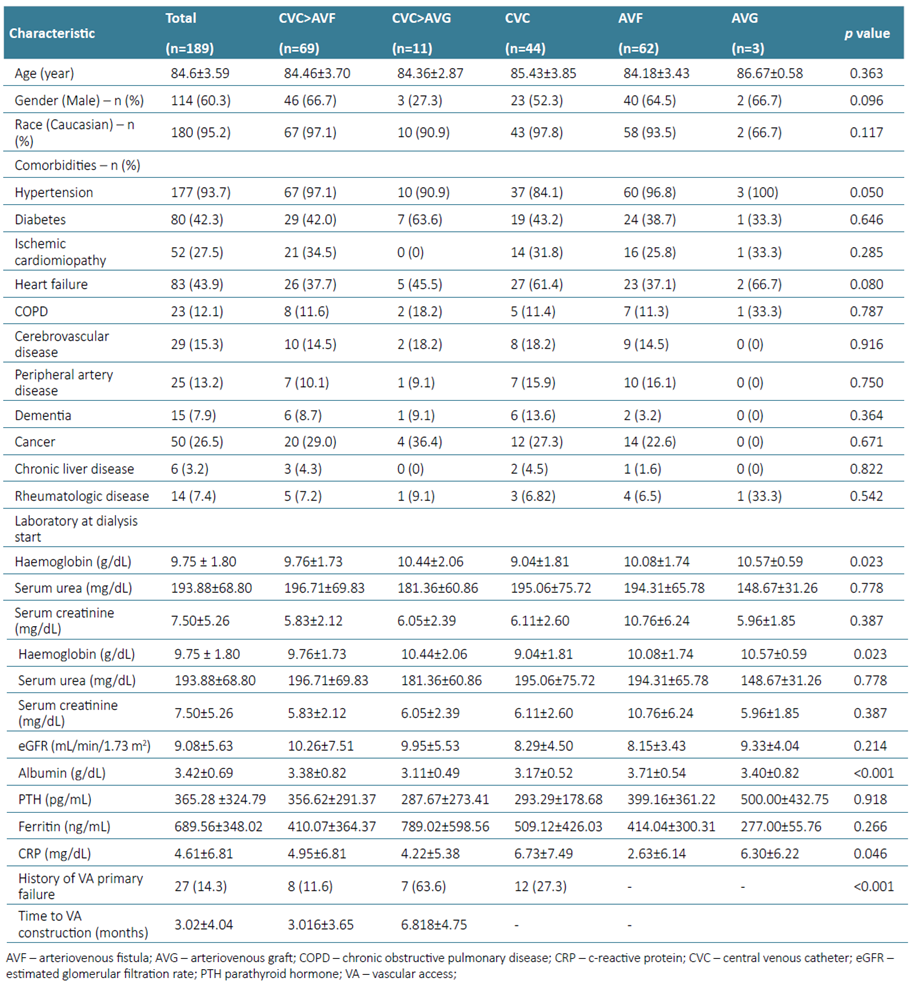
From a total of 787 incident patients on HD as a first technique for RRT at CHULN between January 2014 and December 2019, 189 were ≥ 80 years old at the time of dialysis start and therefore were eligible for the study. The mean age was 84.6 ± 3.59 years and the majority were male (60.3%) and Caucasian (95.2%). The baseline characteristics are depicted in Table 1.
Most patients had a previous diagnosis of hypertension (93.7%) and the overall prevalence of diabetes was considerably high (42.3%). As for other comorbidities, ischemic cardiomyopathy accounted for 27.5% cases, heart failure for 43.9% cerebrovascular disease for 15.3% and peripheral arteriopathy for 13.2%. More than twelve percent of patients had COPD, 7.9% had dementia, 7.4% had rheumatologic disease and 3.2% had chronic liver disease. It is also noteworthy to see that more than one‑fourth of patients (26.5%) had an active cancer at the time of dialysis initiation.
Laboratory workup at HD start included mean hemoglobin of 9.8 ± 1.8 g/dL, eGFR was 9.1 ± 5.6 mL/min/1.73m2, serum creatinine was 7.50 ± 5.26 mg/dL, CRP was 4.6 ± 6.8 mg/dL, albumin was 3.4 ± 0.7 g/dL, serum ferritin was 689.6 ± 348.0 ng/mL, serum PTH was 365.3 ± 324.8 pg/mL and mean urea was 193.9 ± 68.8 mg/dL.
Vascular access at HD start was a CVC in 124 patients (65.6%), whereas 62 (32.8%) started with an AVF and only 3 patients (1.6%) started with an AVG. During follow‑up, from those who started HD through a CVC, 27 (21.8%) had a history of vascular access primary failure. In sixty‑nine (36.5%) patients a functional AVF was created and in 11 (5.8%) an AVG was placed. Forty‑four patients (23.3%) remained with a CVC during follow‑up. The time to construction of a functional AVF or AVG was 3.6 ± 4.0 months. Patients baseline characteristics were similar across all five groups. However, patients who remained with a CVC and who did not have an AVF of AVG created had lower Hb (p=0.023), higher NL ratio (p=0.025), lower serum albumin (p<0.001) and higher CRP (p=0.046) at dialysis start (Table 1).
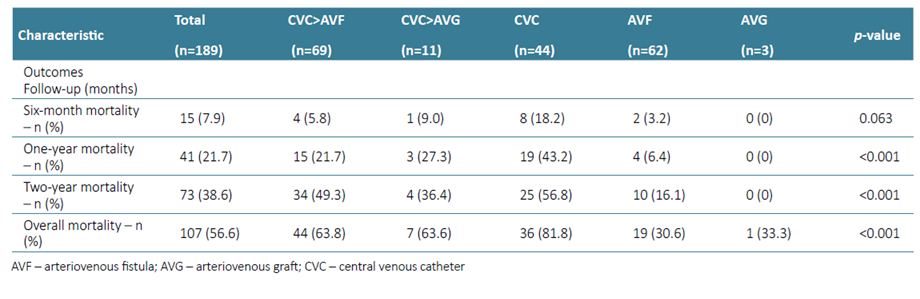
The primary outcome, one‑year mortality, was observed in 21.7% of patients (n=41) (Table 2).
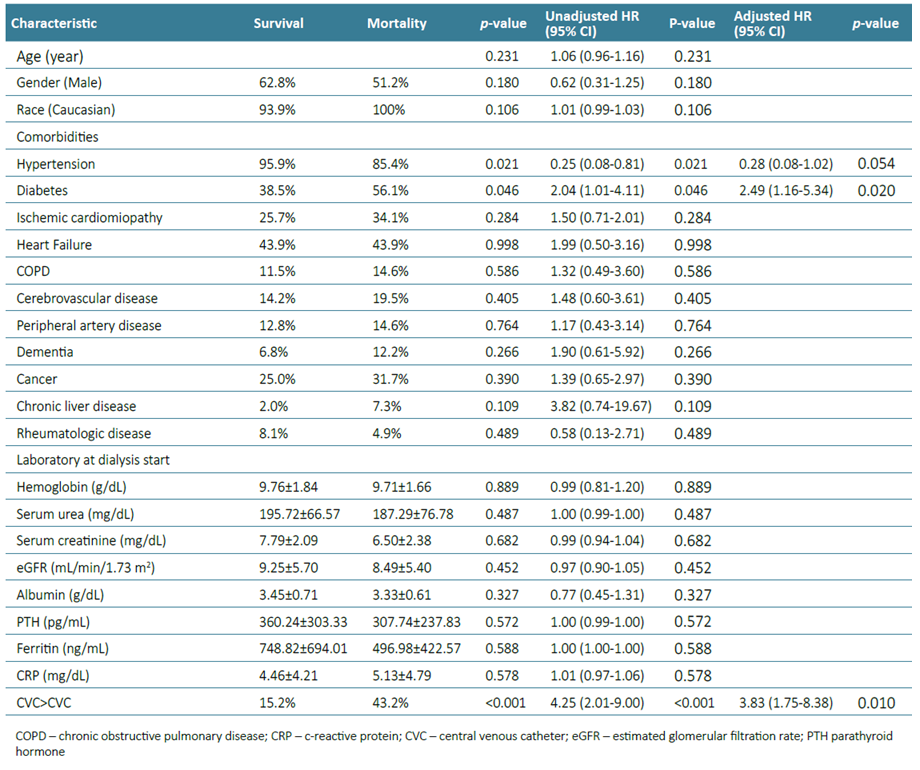
Patients who died within the first year had more frequently diabetes (56.1% vs 38.5%, p=0.044; HR 2.04 (1.01‑4.11), p=0.046), had less frequent hypertension (85,4% vs 96.0%, p<0.014; HR 0.25 (0.08‑0.81), p=0.021). Vascular access at dialysis initiation and during follow‑up was also a detrimental factor for the primary outcome, as patients who remained with a CVC in the first year had the highest mortality (43.2% vs 15.2%, HR 4.25 (2.01‑9.00), p<0.001). At one year, the survival of patients with AVF after CVC, AVG after CVC, remaining with CVC, starting with AVF and starting with AVG was 78.3%, 72.7%, 56.8%, 93.5% and 100% (p<0.001) respectively. No laboratory findings at the beginning of HD were predictive of mortality within the first year (Table 3). On the multivariate analysis, only diabetes (aHR 2.49 (1.16‑5.34), p=0.020) and remaining with a CVC (aHR 3.83 (1.71‑8.38), p=0.001) were significant predictors of one‑year mortality.
Table 3. One‑year mortality according to patients characteristics and univariate and multivariate mortality predictors analysis
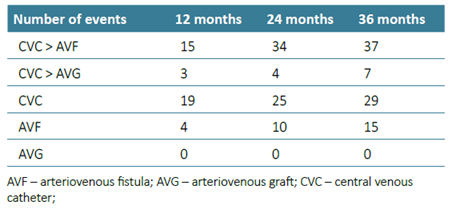
We conducted a sub‑analysis including only patients who started HD with a CVC. Of the 124 patients who started HD with a CVC, construction of an AVF or AVG was attempted in 92. The rate of primary failure was 29.3% (n=27). Remaining with a CVC was an independent predictor factor for one‑year mortality (aHR 2.31 (1.02‑5.22), p=0.045). Kaplan‑Meier analysis revealed that the three‑year survival rates for patients starting with AVG, starting with AVF, with AVF after CVC, starting and remaining with CVC and with AVG after CVC were 100%, 75.8%, 46.4%, 34.1% and 36.4% (p<0.001) respectively (Fig. 1).
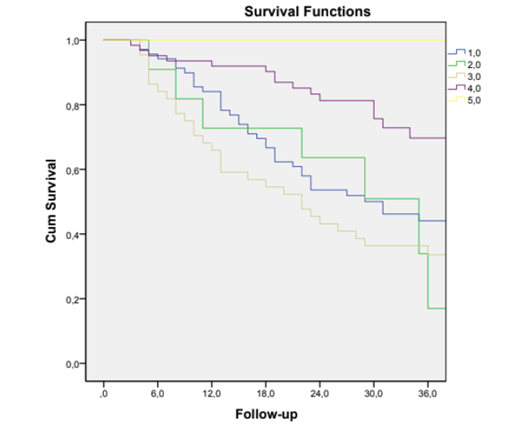
Figure 1. Kaplan‑Meier survival curves of overall survival analysis in patients with different vascular accesses
DISCUSSION
In this retrospective observational study of very elderly patients, we accomplished to demonstrate that starting HD with a CVC and remaining with this VA was associated with higher one‑year mortality rather than starting with or switching to arteriovenous access. This benefit extends to at least three years, with conversion to AVF or AVG after starting with a CVC still conferring less risk for mortality.
Multiple previous studies have demonstrated that patients who start HD with a CVC have lower survival compared to those who start with an AVF or AVG.20‑22 In those who start HD with a CVC, switching to arteriovenous access has also been associated with a substantial decrease in mortality risk (30%‑60% lower adjusted risk of death) as well as other important endpoints such as malnutrition, inflammation and anemia.23‑25 Several other comorbidities such as diabetes, hypertension, and heart failure, as well as laboratory parameters like hemoglobin, albumin and ferritin, have also been shown to be important mortality predictors in HD patients.26‑28 In our study, however, only diabetes was a predictor of mortality and there was a tendency towards hypertension to be protective. This inquiring aspect may be assignable to the fact that comorbidities inducing hypotension, such as multiple myeloma or amyloidosis, may be more frequently associated with mortality. Also, hypotensive patients had more frequent CVC as definitive VA, which could have an impact in the ability to create arteriovenous access. Although in our study, we did not identify other factors, such as malnutrition and inflammation as predictors of mortality, this may be due to the reduced number of patients in the sample. We also highlight the high number of cancer patients, which may also explain the 3‑year mortality.
After adjusting for the comorbidities, starting HD with a CVC and remaining with this VA was an independent predictor of one‑year mortality. Although this may point to the direct contribution of CVCs to patient morbidity, namely catheter‑related infections, hospitalizations and mortality, this association may not be causal, and other factors might impact the outcome. Several other factors should be taken into account, such as previous clinical follow‑up by a nephrologist and planned versus urgent dialysis start, as these factors may also impact on the prognosis. We also hypothesized that the burden of comorbidities may lead to not being proposed or not being able to build a definitive VA, and comorbidities, rather than the VA itself, are the cause of death. This highlights one of the principal pitfalls regarding outcome analysis concerning vascular access: the difference in outcomes between groups with different vascular access is due to the underlying demographic and laboratory characteristics that lead the patients to receive a certain vascular access type rather than the type of vascular access itself.29 In the overall population of HD patients, the increased awareness of the importance of VA as a marker of treatment quality, survival and lower health‑care costs led to the Fistula First Breakthrough Initiative, which aimed to reduce the number of patients with catheters and increase the number of AVFs.30 However, the theoretical benefit of AVFs in the very elderly (patients aged > 80 years old) is somehow debatable. Shorter patient survival, high rates of primary AVF failure and longer maturation time are all factors that must be taken into account when selecting patients for AV access creation, as the access may not predict survival in this subgroup.31‑33 In our cohort, it is note‑ worthy that 74.2% of patients starting dialysis with a CVC had an attempt at construction of arteriovenous access, mainly AVF, and the rate of primary failure was 29.3%. Although our work was not aimed to determine the time until utilization of an AVF or AVG, it is well known that primary failures and the option for an AVF with the need for prolonged (sometimes assisted) maturation entail an extension of time with a CVC, with potential deleterious outcomes.34‑36 Comorbidities, geriatric syndromes such as frailty and dementia, as well as patient’s preferences, must also be considered in the dialysis life plan of these patients37. Bearing this in mind, placing an AVF may not be justified according to the patient’s clinical status or life expectancy and the choice for an AVG, whose primary patency is superior and can be cannulated within a couple of weeks, may be reasonable, as demonstrated by the low one‑year mortality in our study.
Although the present work does not answer the question about which elderly patients benefit from undergoing hemodialysis by catheter and not by arteriovenous access, the authors emphasize that several prognostic tools to estimate survival have been developed and validated in elderly patients in need of dialysis, such as the French Renal Epidemiology and Information Network risk score and the Cohen scale.38,39 In the author’s opinion, elderly patients with a life expectancy of 6 months or less should not be submitted to arteriovenous access construction after starting hemodialysis and the nephrologist must mainly focus on quality of life and patient‑reported outcome measures rather than goals regarding hypertension, anemia, mineral bone disease control, or even the vascular access. Patient comorbidities and the possibility of placing arteriovenous access are also important to investigate as we demonstrated that patients who remained with a CVC have lower survival at one and three years of follow‑up. Major strengths of this study include the size and unselected nature of our population, and the ability to take major potential confounders into account in the multivariate analyses, including numerous comorbidities and laboratory values. The three‑year follow up is also noteworthy since the population in the study had 84.6±3.59 years at dialysis start.
This work has some limitations of which we highlight the retrospective and single‑center nature of the study. Also, we did not differentiate patients according to previous follow‑ups by a nephrologist nor whether dialysis start was planned or emergent. Additionally, comorbidities are coded as present or absent, and not by their severity. For example, two patients may be categorized as having diabetes; however, one has had the disease for 3 years, with good glycemic control and no macrovascular disease and the second has more than 20 years of diabetes, poor glycemic control and severe cardiovascular complications. Also, we report clinical outcomes based on vascular access at dialysis initiation, but updated information on vascular access was not available during follow‑up. Finally, causes of mortality were not assessed.
In conclusion, among elderly patients, initiating and maintaining HD with a CVC is associated with one‑year mortality and with long‑term mortality. Whether the vascular access type affects the clinical outcome or simply acts as a marker for the severity of comorbidities remains unanswered. Further prospective studies could be interesting in enlightening on the ideal vascular access type in the elderly. Quality of life, more than survival, and patient‑reported outcomes must also be regularly included in the analysis of outcomes of older patients in dialysis.