CLINICAL PRESENTATION
A 45‑year‑old man was referred to our hospital due to nephrotic syndrome (NS) and acute kidney injury (AKI) with onset one week after the COVID‑19 vaccine (Ad26.COV2‑S [recombinant]). His previous medical history included ear leiomyosarcoma, excised thymoma and follow‑up free of relapse, two ischaemic strokes, hypertension, obesity (body mass index of 39.7 kg/m2), and obstructive sleep apnea syndrome. A relevant family history of cardiovascular events was identified. His current medication includes enalapril, omeprazole, aspirin, and allopurinol.
Upon admission, he presented with anasarca, and the initial findings from laboratory tests indicated a serum creatinine (SCr) of 2.52 mg/dL (baseline 0.7 mg/dL), severe hypoalbuminemia (1.98 g/dL), and 24h‑urine albuminuria (24h‑UA) of 13.3 g/24h. Abundant lipid content was observed in the urinary sediment. Kidney ultrasound findings were unremarkable and a kidney biopsy was performed showing 5 glomeruli with no relevant alterations in light microscopy and with negative immunofluorescence. A computed tomography scan excluded a neoplastic cause and other causes (such as drugs, infections, and autoimmune diseases) were ruled out.
Prednisolone (PDN) was started (1 mg/kg/day) and a partial response was observed in the following 16 weeks with a SCr level of 0.96 mg/dL, albuminuria of 3.1 g/dL and 24h‑UA of 2.8 g/24h.
Six months later, the patient relapsed (anasarca, hypoalbuminemia 2.3 g/dL and 24h‑UA 11 g) two weeks following the COVID‑19 infection diagnosis. He was readmitted for hospitalization and another kidney biopsy was performed (Fig. 1).
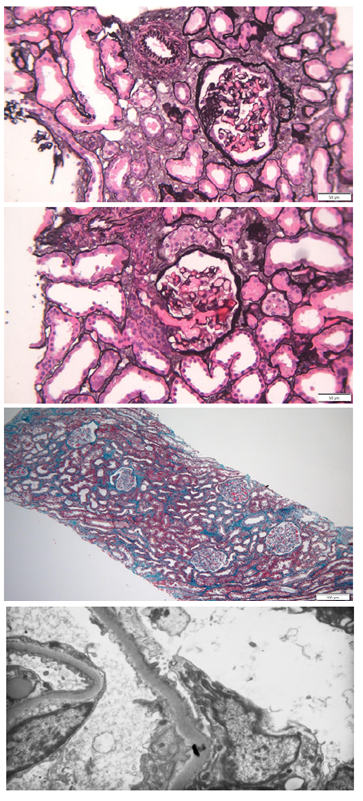
Figure 1. Kidney biopsy findings. (A) Methenamine silver staining; original magnification. (B) Methenamine silver staining; original magnification. (C) Masson’s trichrome staining; original magnification. (D) Diffuse podocyte effacement on electronic microscopy
What is the differential diagnosis of this patient’s presentation?
Our patient presented with NS after COVID‑19 immunization and relapsed after COVID‑19 infection, both of which are documented presentations in existing literature.1 The reported instances highlighted NS in middle‑aged patients, typically manifesting around seven days after the triggering event and involving severe proteinuria.1 Considering the diagnosis of podocytopathies, the presentation as sudden‑onset NS favors a permeability factor‑mediated etiology, although hyperfiltration could also contribute considering the patient’s obesity and hypertension background.2 Focal segmental glomerulosclerosis (FSGS) was the most frequent histologic pattern reported in patients who presented with NS after COVID‑19 immunization or infection,1 although membranous nephropathy and minimal change disease (MCD) were also found.1,3
The first kidney biopsy result favored MCD diagnosis, although FSGS could not be excluded due to the focal character of the lesions and the reduced number of glomeruli in the sample. This uncertainty prompted the consideration of a second kidney biopsy following the patient’s relapse.
A summary of glomerular patterns and their causes, pertaining to the patient’s differential diagnosis, is presented in Box 1.
What does the kidney biopsy show?
The kidney biopsy was composed of 42 glomeruli, out of which 2 were globally sclerosed and 4 had FSGF lesions (Fig. 1A). Hypertrophy was observed in most of the glomeruli (Fig. 1B).
The interstitium had edema without significant fibrosis and severe acute tubular necrosis was observed (Fig. 1C).
How should this patient be managed?
Given the sudden NS presentation and the findings of the first kidney biopsy, MCD was the diagnosis considered and PDN was started with partial response.
After the first relapse and the second diagnosis showing FSGS, cyclosporine (CSA) was started due to partial response to PDN as first‑line but also as a glucocorticoid sparing regimen for our patient.4 The use of calcineurin inhibitors in podocytopathies relies on a relatively small number of studies with limited follow‑up, and the ultimate renal and broader outcomes remain uncertain.5 Approximately two months after the initiation of CSA, a new relapse occurred with no identified trigger. The patient was readmitted with anasarca and 24h‑UA of 18 g. The levels of CSA were within the appropriate range.
Given the risk of immunosuppression exposure in a patient with a heavy neoplastic background and the relapsing behavior of the disease, genetic testing was conducted to account for potential immunosuppression‑related risks. No pathogenic variations were found in the genetic testing. In light of the incomplete response to both steroids and CSA, the decision was made to initiate rituximab treatment. The regimen consisted of 1 g administered on both day 0 and day 15, followed by a maintenance dose of 1 g every 6 months. This treatment approach was pursued over a 12‑month follow‑up period, resulting in the resolution of nephrotic syndrome with no relapses.
In addition to the immunosuppressive treatment, enalapril was introduced to the maximum tolerated dose (7.5 mg/day) as well as spironolactone (25 mg/day) and dapagliflozin (10 mg/day). The patient was also directed to a nutritional consultation, where guidance was provided to adopt a healthy diet and enhance physical activity.4 In the last follow‑up appointment (2 years after diagnosis), the patient was euvolemic and his laboratory workup showed a SCr level of 1.3 mg/dL, and a 24h‑UA 0.2 g.
Recognizing the significance of treatment‑resistant or frequently recurring NS in terms of mortality risk and the potential advancement to end‑stage kidney disease,5,6it becomes imperative to implement a comprehensive strategy for the patient. This approach should encompass all relevant factors that might contribute to glomerular damage.