WHAT’S ALREADY KNOWN ABOUT THISTOPIC?
Calciphylaxis is a life‑threatening vasculopathy resulting from calcium deposition in the arteriolar microvasculature of the deep dermis and subcutaneous adipose tissue. It is rare in end‑stage kidney disease (ESKD) treated with maintenance dialysis and even rarer in patients without ESKD, with no consensus regarding its nomenclature: calcific uremic arteriolopathy in ESKD, nonuremic calciphylaxis before ESKD or calciphylaxis related to each chronic kidney disease (CKD) stage. However, different classifications are associated with different prognoses. This ambiguity parallels the challenges in calciphylaxis diagnosis and directed treatment initiation.
WHAT DOES THIS STUDY ADD?
This case report gives insight into the possibility of calciphylaxis in a pre‑dialysis CKD setting without other significant classical risk factors for the disease and into the change in prognosis with early therapy initiation.
LEARNING POINTS/TAKE HOME MESSAGES:
Calciphylaxis is a rare disease with an ominous prognosis that can occur in any stage of CKD. Calciphylaxis risk factors may not be present, so clinical suspicion must be high. Early directed therapy initiation and a multidisciplinary approach are essential for mortality reduction.
INTRODUCTION
Calciphylaxis is a rare life-threatening vasculopathy resulting from calcium deposition in the arteriolar microvasculature of the deep dermis and subcutaneous adipose tissue.1 Calciphylaxis is classically associated with chronic kidney disease (CKD), also referred to as calcific uremic arteriolopathy (CUA). It mainly occurs in end-stage kidney disease (ESKD) treated with maintenance dialysis,2 although only 1%‑2% of all ESRD patients develop calciphylaxis.3 However, calciphylaxis may also occur in patients without ESKD, known as nonuremic calciphylaxis (NUC).4 ESKD is one of the most important risk factors for calciphylaxis development. Other notable risk factors include female sex, obesity, diabetes mellitus, warfarin use, corticosteroids use, calcium and vitamin D supplements overuse, vitamin K deficiency, hypercoagulability states such as protein C and S deficiencies, Crohn disease, autoimmune disorders such as antiphospholipid syndrome, substantial weight loss, recurrent hypotension, malignant neoplasms (cholangiocarcinoma, hematologic malignancies, and melanoma), hyperparathyroidism, vitamin D deficiency,1 calcium-phosphate product over 70 mg2/dL2, and serum aluminium greater than 25 ng/mL.2 Calciphylaxis has a high risk of mortality, with an estimated 1‑year survival rate of calciphylaxis between 45.8% and 50%,2,5,6with sepsis from infection of cutaneous wounds being the leading cause of death in these patients.2 CUA is associated with higher mortality when compared with NUC.7We present a clinical case of a stable stage 5 CKD patient who developed skin lesions in whom calciphylaxis was not initially suspected because several common risk factors were not present, such as maintenance dialysis, altered calcium‑phosphate product or warfarin use.
CASE REPORT
A 75-year-old female presented to the emergency department due to worsening pain and increasing size of a right lower limb skin lesion. The patient had a medical history of CKD due to diabetic kidney disease for >15 years, slowly progressing to pre‑dialytic stage 5 CKD (basal estimated glomerular filtration rate through CKD‑EPI creatinine [2021] equation of 10‑15 mL/min/1.73 m2), chronic anaemia, secondary hyperparathyroidism (for >3 years), type 2 diabetes with target organ damage (although with adequate glycaemic control), arterial hypertension, hyperuricemia, overweight (body mass index 28.6 kg/m2), heart failure (HF), and chronic venous insufficiency. She was medicated with epoetin beta 2000 units biweekly, ferrous sulphate 329.7 mg per day, cholecalciferol 3500 units per day (for >2 years), calcitriol 0.25 mcg triweekly (for >1 year), basal insulin 18 units daily, rilmenidine 1 mg twice a day, nifedipine 30 mg per day, bisoprolol 2.5 mg per day, furosemide 80 mg per day, clopidogrel 75 mg per day, allopurinol 150 mg per day, lansoprazole 30 mg per day, and tramadol 50 mg twice a day. One month prior, the patient was admitted to the hospital due to decompensated HF, where an ulcerated lesion on the posterior surface of the right lower limb was identified for the first time, interpreted as an infected varicose ulcer, and treated with amoxicillin/clavulanic acid. In the current episode, she went back to the emergency department due to worsening pain and the increasing size of the skin lesion. On physical examination, there was an ulcer on the posterior surface of the right leg with reticulated contours, a fibrin‑necrotic background and erythematous edges, without infiltration of its base and without surrounding livedo reticularis, as well as new lesions in both lower limbs, particularly in the legs and feet (Fig. 1). The diagnoses of ulcers due to vascular micro-occlusion secondary to calciphylaxis, cholesterol embolism or oxalate vasculopathy were hypothesized. The analytical study revealed elevated serum creatinine (2.92 mg/dL), blood urea nitrogen (69 mg/dL), sedimentation rate (80 mm/h), parathyroid hormone (PTH, 394 pg/mL), and phosphorus (5.3 mg/dL, without altered calcium‑phosphate product (46.4 mg²/dL²); autoimmune pathology and thrombophilia were excluded (Table 1). A skin biopsy was performed, and the patient was discharged.
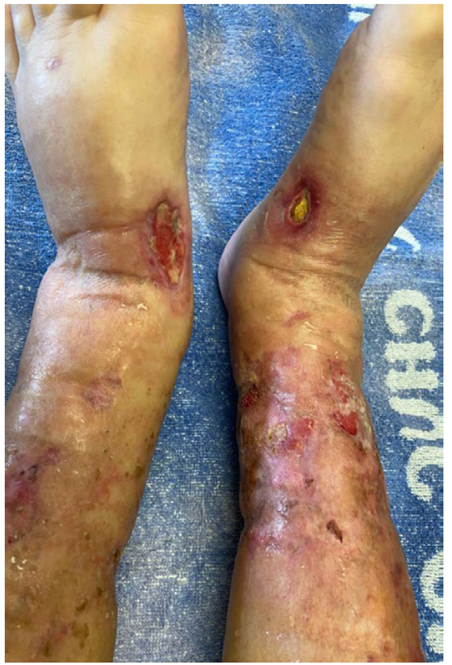
Figure 1 Skin ulcers with reticulated contours, a fibrin‑necrotic background and erythematous edges, without infiltration of its base and without surrounding livedo reticularis.
Skin histologic analysis (Fig. 2) revealed ulceration, partial necrosis on the surface, marked vascular congestion, and multiple calcifications, some extravascular and others reaching the wall of small and medium‑sized vessels; these findings were compatible with calciphylaxis. Before directed therapy could be started, the patient was readmitted to the hospital one month later due to worsening calciphylaxis lesions and associated bacterial superinfection with progression to sepsis. Antibiotic therapy was performed with different agents (levofloxacin, linezolid, clindamycin, meropenem, flucloxacillin, and piperacillin/ tazobactam), analgesia adjustment due to severe pain associated with the skin lesions (gabapentin, metamizole, buprenorphine, and fentanyl), and sodium thiosulfate was started, with clinical improvement of the lesions. Despite the implemented measures, the patient presented worsening renal function (serum creatinine: 5.27 mg/dL), leading to haemodialysis (HD) initiation. She continued triweekly HD treatment with the administration of 25 g of intravenous sodium thiosulfate in each dialysis session and follow‑up at a dermatology and pain outpatient clinic. Wound care was provided with Aquacel® Ag+ antimicrobial dressings.
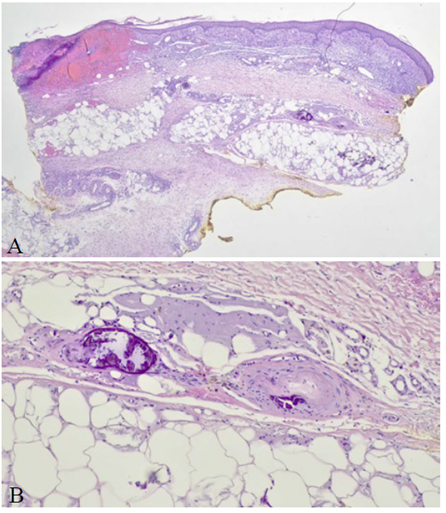
Figure 2 Histologic examination on optic microscopy of skin tissue sample. Haematoxylin and eosin staining. A. Original magnification 20x, ulceration and partial necrosis on the surface (asterisk), marked vascular congestion, and multiple calcifications (small arrows). B. Original magnification 200x, vascular calcification reaching the wall of a medium-sized vessel (large arrows).
Re‑epithelialization of the ulcerated lesions was achieved after one year of therapy without new episodes of super-infection, so sodium thiosulfate was discontinued. Four months after drug discontinuation, there was a relapse of the skin lesions, and therapy was rebegun for three months, with complete healing of the lesions and no relapse for over one year of follow‑up. No other wound‑directed therapy was needed, such as debridement, negative‑pressure wound therapy or hyperbaric oxygen therapy (HBOT).
DISCUSSION
The diagnosis of calciphylaxis may be challenging. In patients with CKD, there should be high clinical suspicion of calciphylaxis after the appearance of painful nodules, indurated plaques, dusky livedoid plaques, or nonblanching retiform purpura. Calciphylaxis should still be suspected even if such lesions appear in sites other than the abdomen and proximal lower limbs.8 Of note, retiform purpura is a cutaneous morphology that commands a broad differential diagnosis; clinicopathologic correlation and judicious workup are necessary to rule out clinical mimickers.9 The most frequent initial diagnoses are cellulitis (31.0%), unspecified skin infection (8.0%), and peripheral vascular disease (6.9%).10 In the presented clinical case, the same challenges arose, and the patient was initially started on antibiotics on the suspicion of an infected varicose ulcer. This led to a delay of two months in the correct diagnosis (biopsy-proven) and directed therapy, leading to a related complication with lesion superinfection and sepsis. In this case, calciphylaxis can be classified as CUA, although there are some nuances regarding this matter. The definition of CUA and NUC is variable in the literature, and this clarification is of utmost importance since the first has a worse prognosis. For some authors, NUC is defined for any altered kidney function besides ESKD,4 while in other studies, NUC was only considered when a normal kidney function was present.11 We tend to agree with the McCarthy study, which did not classify calciphylaxis as CUA or NUC but divided the patients in relation to their CKD stages. To justify this division, McCarthy stated that the lower survival in patients with CKD suggests that CKD may directly add more risk or may imply different risk factors in patients with and without CKD.6 Furthermore, Nigwekar’s recent study stated that since calciphylaxis can occur in all CKD stages, they prefer the broader entity calciphylaxis to CUA.1 So, we can say that our patient had the diagnosis of calciphylaxis related to stage 5 CKD in progression to ESKD with the need for maintenance dialysis. Regarding other risk factors, the predictive value of serum markers such as serum calcium, phosphate, PTH, and albumin levels in the development of calciphylaxis remains an important question.12,13Theoretically, these parameters could be useful in modifying therapies and HD strategies for patients at a higher risk of developing calciphylaxis.
However, many patients with calciphylaxis may have an unremarkable calcium‑phosphorus product.2A recent analysis of data from the German Calciphylaxis Registry showed that 86% of dialysis‑dependent patients with calciphylaxis had either normal or low plasma calcium levels, and 40% had either normal or low plasma phosphate levels.14Further studies are needed to clarify if laboratory markers may be of diagnostic or risk‑stratification utility.8 In the presented clinical case, besides CKD, we can also find other culprit factors: female sex, overweight, diabetes mellitus, elevated PTH and phosphorus, despite a normal phospho-calcium product,15iron and vitamin D supplementation, and a less frequently implicated but possible factor, furosemide use.11 We highlight the importance of considering all risk factors in patients with ESKD who are not on maintenance dialysis because a cumulative association of risk factors may be important for developing calciphylaxis. Skin biopsy is the standard method for the confirmation of clinically suspected calciphylaxis. The relationship between the Koebner phenomenon (i.e. the appearance of new skin lesions on previously unaffected skin secondary to trauma) and calciphylaxis highlights the importance of obtaining a punch biopsy in these patients. Punch biopsy rather than excisional biopsy is recommended to avoid nonhealing wounds, even though it may not yield adequate tissue depth.16 Histopathologic findings may be subtle, and special staining (i.e. von Kossa) may aid in identifying stippled calcifications of small subcuticular vessels.17 On histopathology, calciphylaxis is characterized by calcification of small vessels (<100 μm) in the deep dermis and subcutaneous tissue, along with fibrin thrombi and, occasionally, evidence of ischemic epidermal and dermal necrosis. These findings are distinct from other forms of vessel calcification.18 Despite the importance of the histologic diagnosis, a biopsy is not needed for a patient with ESRD and the classic presentation of a painful necrotic ulcer covered with a black eschar.1 Calciphylaxis treatment is a real challenge since all available data is gathered through case series, which, due to calciphylaxis rarity, are very valuable as a tool for disseminating successful outcomes in novel therapies. Multipleagent approaches in conjunction with HD have been reported to be efficacious. Management of calciphylaxis, whether uremic or non‑uremic, begins with stopping all medications that increase the risk of calciphylaxis, namely, warfarin, iron, and calcium‑containing medications. HD therapy is expected to be beneficial since it improves mineral abnormalities and accelerates wound healing. Careful wound care is also beneficial. Surgical debridement accompanied by negative‑pressure wound therapy is recommended for infected wounds and large necrotic areas with drainage. However, such therapy can result in defective soft tissue lined by marginally viable tissue, requiring further excision.1 HBOT has been studied in calciphylaxis and appeared to improve outcomes in more than half of the patients in a series of 34 CUA patients who received a full course of HBOT.19 Narcotics are generally required for pain control, and some authors prefer fentanyl over morphine owing to the decreased risk of hypotension with the former agent. Sodium thiosulfate is the most commonly and specifically used agent to manage calciphylaxis (both CUA and NUC). As many as 70% of patients with calciphylaxis respond favourably to sodium thiosulfate therapy. This agent is an effective calcium chelator in vitro. Notwithstanding, it may operate through other mechanisms in calciphylaxis, as studies have shown that the calcium-phosphate product is not normalized even in patients who respond well to thiosulfate therapy. In patients with eGFR >60 mL/min/1.73 m2, the usual sodium thiosulfate dose is 25 g administered intravenously up to 5 times per week.20 For HD patients, the preferred regime is 25 g (100 mL of a 25% solution) administered intravenously triweekly during the last 30-60 min of each HD session. This last approach is relatively safe, has satisfactory outcomes, and is well tolerated by patients for short to medium treatment duration.1 In our patient, triweekly therapy with 25 g of intravenous sodium thiosulfate was the chosen approach with excellent results in a patient who had a presenting episode of sepsis related to the skin lesions superinfection, which is the leading cause of death in these patients.2 Such success is expressed in the patient’s complete wound healing after almost three years from initial presentation in a disease with an estimated 1‑year survival rate of calciphylaxis between 45.8% and 50%.2,5,6An interdisciplinary approach, including specialists in dermatology, nephrology, nutrition, pain, palliative medicine, plastic surgery, and wound care, to expedite the diagnosis and treatment, is the core for the best care of patients with calciphylaxis.1 In conclusion, calciphylaxis is rare and even rarer in patients not on maintenance dialysis. The threshold of suspicion must be high in patients with CKD, even without other common risk factors, as shown in this clinical case. Early identification and directed therapy initiation may be lifesaving in a disease that portends an ominous prognosis.