Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Análise Social
versão impressa ISSN 0003-2573
Anál. Social no.205 Lisboa dez. 2012
The wandering mind: Mental time travel, theory of mind, and language
O espírito errante: Viagens mentais no tempo, teoria da mente e linguagem
Michael C. Corballis*
*Department of Psychology, University of Auckland. E-mail: m.corballis@auckland.ac.nz
Abstract
Mental time travel includes the ability to bring to mind past events (episodic memory) and imagine future ones. Theory of mind is the ability to understand what others are thinking or feeling. Together, these faculties are dependent on the so-called default mode network in the brain, which is active when the mind is not engaged in interaction with the immediate environment. They enable us to mentally escape the present, and wander into past and future and into the minds of others. Language evolved out of gestural systems, probably during the Pleistocene, to enable people to share their mind wanderings, and tell stories, including fictional ones.
Keywords: Default mode network; episodic memory; future thinking; gesture; hippocampus; language; theory of mind.
Resumo
A viagem mental no tempo inclui a capacidade de relembrar acontecimentos passados (memória episódica) e de imaginar eventos futuros. A teoria da mente é a capacidade de compreender aquilo que os outros estão a pensar ou sentir. Juntas, estas capacidades dependem do chamado default mode network cerebral, que se encontra ativo quando a mente não se encontra implicada em qualquer interação com o ambiente circundante imediato. Estas capacidades permitem-nos escapar mentalmente ao presente e vaguear pelo passado, pelo futuro, e pelas mentes dos outros. A linguagem evoluiu a partir de sistemas gestuais, provavelmente durante o Pleistoceno, de modo a permitir ao ser humano partilhar as suas deambulações mentais e contar histórias, inclusive histórias ficcionadas.
Palavras-chave: Default mode network; memória episódica; pensamento futuro; gesto; hipocampo; linguagem; teoria da mente.
INTRODUCTION
Throughout history it has been widely assumed that the human mind far surpasses that of other animals in both degree and kind. Language, in particular, has seemed so unconstrained and open-ended that it must have been a divine gift, as indeed was declared in the Bible: In the beginning was the Word, and the Word was with God, and the Word was God (John 1, p. 1). This view was given philosophical respectability by the 17th-century philosopher René Descartes, who established a modern tradition of argument for the special, God-given nature of human thought. He was much interested in mechanical toys, and asked whether human thought might itself be reduced to mechanistic principles. It was the sheer flexibility of language, he argued, that seemed to preclude this possibility, and in a 1646 letter to the Marquess of Newcastle, he wrote that:
[ ] none of our external actions can show anyone who examines them that our body is not just a self-moving machine but contains a soul with thoughts, with the exception of words, or other signs that are relevant to particular topics without expressing any passion.
In accordance with biblical writing, then, language and free will must have been gifted by God. The challenge to create mechanical models of the mind is still with us among those working in artificial intelligence or robotics, with at best limited success in such operations as pattern recognition, language processing, or goal-directed movement. There has been virtually no progress toward such elusive concepts as free will, and even language remains only partially understood.
Modern scholars are less inclined to invoke divine intervention, but nevertheless argue that language, in particular, must have been the result of some event, perhaps a fortuitous mutation, that made the human mind unique. The linguist Noam Chomsky, for instance, has argued that this momentous event took place within the past 100,000 years, endowing us with a mechanism he describes as unbounded Merge, creating the recursive processes underlying the open-ended structure of language as well as of thought itself. He recently wrote as follows:
Within some small group from which we are all descended, a rewiring of the brain took place in some individual, call him Prometheus, yielding the operation of unbounded Merge, applying to concepts with intricate (and little understood) properties [Chomsky, 2010, p. 59].
Chomsky finds additional support for this great leap forward, as he calls it, from archaeology. It has long been held that what has also been termed a human revolution (e.g., Mellars and Stringer, 1989) occurred at some point within the past 100,000 years, well after the emergence of our own species some 160,000 to 200,000 years ago. It was marked by dramatic increases in the sophistication of tools, bodily ornamentation, cave art, statuettes, burial rites, and even music. For a time it was thought that these developments, heralded as the beginnings of modernity, were confined to Europea view that may echo the Victorian notion that Europeans, and males in particular, constituted the highest form of humanity. Evidence now suggests that the great leap forward can be traced to Africa, before the human dispersals around 60,000 years ago that led eventually to human presence around the globe (Mellars, 2006). At least some of the markings of modernity are evident not only in Africa itself, but also in other endpoints of the original dispersals, such as Australia and New Guinea.
The archaeologist Richard Klein, though, has argued that the great leap forward may have been as late as 50,000 years ago (50 ka), and he is one of those who continue to hold that it was initially confined to Europe. He writes that it is at least plausible to tie the basic behavioral shift at 50 ka to a fortuitous mutation that created the fully modern brain (Klein, 2008, p. 271). Another archeologist, John Hoffecker, writes similarly, although he links the change specifically to language and suggests an even more recent date:
Language is a plausible source for sudden and dramatic change in the archaeological record [after 40 ka] because: (a) it is difficult to conceive of how the system for generating sentences (i.e., syntax) could have evolved gradually, and (b) it must have had far-reaching effects on all aspects of behavior by creating the collective brain [Hoffecker, 2007, p. 379].
There is nevertheless disagreement as to whether there was truly a discontinuity at all. McBrearty and Brooks (2000) write of the revolution that wasnt, arguing that the advance of human culture was more or less continuous from the Middle Stone Age, around 200,000 to 300,000 years ago. But perhaps it was even earlier. According to evolutionary psychologists, it was the Pleistocene, dating from around 2.6 million years ago to some 12,000 years ago, that supplied the environment of evolutionary adaptiveness (EEA) during which human cognition evolved (Tooby and Cosmides, 1992). Cultural and technological progress increases exponentially rather than linearly, and it is all too easy to see discontinuities in an upwardly accelerating curve; modern observers might be tempted, for example, to see the emergence of the digital computer as the great leap forward of modern times. There is perhaps also a temptation to attribute some special quality to our own species that sharply differentiates us from other animals, if only to justify the manner in which we exploit them.
The notion of a mutation or evolutionary event creating cognitive features as novel as language and free will also flies in the face of the theory of evolution by natural selection. In 1859, Darwin wrote:
If it could be demonstrated that any complex organ existed, which could not possibly have been formed by numerous, successive, slight modifications, my theory would absolutely break down. But I can find no such case [Darwin, 1859, p. 158].
Chomsky has often referred to the language organ, so his view that language and human thought evolved in a single step might perhaps be the case that Darwin sought.
In this article, though, I try to align with Darwinian thinking and argue that the special characteristics of human thought were shaped incrementally, probably with roots in primate evolution, but with elaboration during the Pleistocene, dating from some 2.6 million years ago. I also argue, contrary to Chomsky, that language and thought are distinct, and probably have different though intertwined evolutionary trajectories. I begin with a discussion of a network that may contain the essence of much of human cognition.
THE DEFAULT NETWORK
If people are left to think for themselves undisturbed, without focusing on the immediate environment, their minds wander. These internal thoughts, according to Buckner, Andrews-Hann and Schacter (2008), include autobiographical memory retrieval, envisioning the future, and conceiving the perspectives of others (p. 1). Brain-imaging studies show that mind-wandering activates a widespread network in the brain (see Figure 1), first identified and described as a default network by (Raichle et al., 2001), in which the frontal and parietal lobes play a major role. It is of interest that this network was revealed by subtracting activation during active tasks from that under passive conditions in which subjects were free to let their minds wander (Buckner and Vincent, 2007). That is, task-related behavior essentially deactivates the brain regions involved in the default network. The wandering mind is stopped in its tracks.

A network homologous to the default network in humans has also been mapped out in monkeys, using functional connectivity analysis (Vincent et al., 2007). This raises the question of whether mind wandering is a general feature of the primate brain, and is contrary to claims of human uniqueness, to be discussed below.
MENTAL TIME TRAVEL
Perhaps the most ubiquitous aspect of mind wandering is mental time travel, which combines autobiographical memory retrieval with envisioning the future. The concept of mental time travel was based initially on a distinction, drawn by Tulving (1972), between two forms of explicit (or conscious) memory. Semantic memory is our vast storehouse of facts about the world, whereas episodic memory records specific events. Semantic memory, then, may be likened to an encyclopedia, while episodic memory is like a personal diary. Both are regarded as forms of what has been called declarative memoryor memory that can be declaredwhich already suggests a connection with language. Episodic memory, unlike semantic memory, implies a mental journey into the past, as when one mentally relives or imagines some past episode. Tulving (1972, 1985) proposed that episodic memory is unique to humans.
Episodic memory is notoriously unreliable and incomplete, and it has been proposed that its primary function was not to serve as a faithful record of the past, but rather to provide a basis for imagining and planning of future events. That basis comes in part from memory for particular people, places, and situations, but also from past sequences of actions, or scripts, which can be blended with novel combinations to create plausible future episodes (Bar, 2009). The notion of generating future episodes based in part on past ones led to the notion of mental time travel, implying a continuity of time from past through present to future, and the notion that we can mentally traverse the time dimension (Suddendorf and Corballis 1997, 2007). Continuity is implied by the finding that patients with amnesia typically have as much difficulty imagining future episodes as in recalling past ones (e.g., Hassabis, Kumaran, and Maguire, 2007). Brain imaging reveals that remembering the past and imagining the future activate a common core network (e.g., Addis, Wong, and Schacter, 2007; see Figure 2), which indeed overlaps considerably with the default network, and might be considered part of it. We can of course mentally distinguish past events from imagined future ones, and activation also reflects this. One structure common to both is the hippocampus, but imagining future events activates more anterior regions while remembering past events activates more posterior ones (e.g., Szpunar, Watson, and McDermott, 2007).

Mental time travel into the future does not of course imply precognition in the telepathic sense, but refers rather to the ability to imagine possible future episodes, whether for the purpose of planning detailed activities or for comparing and evaluating different strategies. Such episodes may of course fail to eventuate, or turn out in ways contrary to what was planned. Episodic memory effectively provides the vocabulary of scenarios that enable us to generate and envisage particular future scenarios; its survival value must lie, not in the memory component per se, but rather in what it contributes to present and future survival.
The imagining of events need not always be linked to reality, or even located at specific points in time. We are also compulsively addicted to the once upon a time, as in story-telling, soap operas, movies, myths of heroes and demons. The construction of mental episodes, whether plans for actual future events or the construction of purely imaginary ones, might be seen as a form of mental play, as important for mental life as physical exercise is for physical life. This theme has been explored in relation to fiction by Boyd (2009).
The question of whether mental time travel is uniquely human, as claimed by Suddendorf and Corballis (1997, 2007), has proven highly contentious. A serious challenge has come from studies from a number of nonhuman species, including birds. For instance, scrub jays can recover cached food on the basis not only of where it was cached, but also of when it was cached, which might be taken to imply episodic memory of the caching episode itself (e.g., Clayton, Bussey, and Dickinson, 2003). Jays also appear to cache particular food items based, not on present hunger, but on the basis of what they have been led to expect to have access to on the following day (Correia, Dickinson, and Clayton, 2007). Chimpanzees have been shown to select tools for future use (Osvath and Osvath, 2008) or to collect stones to be later thrown at visitors to the zoo (Osvath, 2011). In these and other studies there are methodological issues, and questions as to whether the results can be interpreted in terms of associative learning rather than the imagining of actual past or future events (see Suddendorf and Corballis, 2007 for a critique).
Perhaps more compelling evidence comes from the hippocampus. In humans, as we have seen, imagining past episodes or imagining future ones both result in hippocampal activity (Addis et al., 2007). In animals, the hippocampus contains so-called place cells, which respond when animals are located in specific spatial locations, as in a maze. In rats, at least, these cells sometimes fire when the animal is outside the maze, either asleep or awake but immobile, and suggest activity associated with activity in the maze. Sometimes this activity corresponds to a previously taken path in the maze, but sometimes to the reverse of such a path, or even to a path the rat did not actually take (Gupta et al., 2010). These findings might be taken as evidence for mental time travel, as though the animal is imagining a previous path or a possible future one (Corballis, 2013).
Human mental time travel, though, goes well beyond the imagining of locations, or the specific contexts of food caching and instinctive behaviors. Premack (2007) suggests that planning for the future is complex, unlike that in the nonhuman species studied to date. Of human planning, he writes:
It is social: two or more individuals form the plan, and the beneficiary of the plan is likely to be yet another individual, different from those who form the plan; the plan is not one-shot, but a series of plans; the plan extends not for hours but over years. Neither social nor sequential planning, nor planning that extends over long durations, is likely to be found in animals [Premack, 2007, p. 13683].
Of course some examples of future-oriented behavior in animals, such as migrations in birds or dam building in beavers, do have fairly long-term consequences, and a degree of complexity. But such behaviors are instinctive, and confined to single activities. Again, they lack the extraordinary flexibility and freedom from context evident in the plans made by humans. Nevertheless human mental time travel may well have evolved from much simpler mechanisms, perhaps involving the hippocampus, and present in present-day animals and birds.
THEORY OF MIND
We wander mentally not only in time, but also into the minds of others. We often knowor think we knowwhat others think or believe. This is known as theory of mind. It is commonly assessed in children through the so-called Sally-Anne Test. The child is shown two dolls, one called Sally and the other called Anne. Sally has a basket and Anne has a box. Sally then puts a marble in her basket and leaves the scene. While Sally is away Anne takes the marble out of the basket and puts it in her box. Sally then comes back, and the child is asked where she will look for her marble. Children under the age of four typically say she will look in the box, which is where the marble actually is. Older children will understand that Sally did not see the marble being shifted, and will correctly say that Sally will look in the basket. They understand that Sally has a false belief (Wimmer and Perner, 1983).
Curiously, an analogous task shows infants as young as seven months to look more quickly and longer at the location where an observer mistakenly believes a ball to be placed than at where it is actually placed (Kovács, Téglás and Endress, 2010). In these respects their behavior does not differ from that of adults. This result might be taken to mean that humans are born with some implicit appreciation of what is in the minds of others, or at least acquire it very early, but cannot articulate this knowledge verbally until much later.
But what of nonhuman species? In 1978, Premack and Woodruff raised the question, Does the chimpanzee have a theory of mind? They were themselves equivocal as to the answer, and their question has led to a long and at times bitter controversy. In a critical examination of the evidence, Penn and Povinelli (2007) conclude that there is no evidence that nonhuman animals, chimpanzees included, have anything resembling a theory-of-mind system. In contrast to the abundant evidence for theory of mind in young children, they claim, the evidence from chimpanzees can be explained in terms of the animals behavioral observations of other animals, rather than an understanding of what is happening in their minds. They also remark that there are good reasons for believing that the traditional hallmarks of human cognition, language and culture, are intimately dependent on [theory-of-mind] systems of various kinds (Penn and Povinelli, 2007, p. 741).
In another review, though, Call and Tomasello (2008) conclude that 30 years of subsequent research have shown chimpanzees to have some understanding of the goals, intentions, perceptions, and knowledge of others, but no understanding of the beliefs and desires of others. From a Darwinian perspective, this conclusion again suggests a degree of continuity, but with greater complexity in humans. The difference may lie partially in levels of recursion (Corballis, 2011). To the extent that chimpanzees can take the perspective of others, they may be said to possess first-order recursion. Tomasello (2008), though, has suggested that humans may be capable of at least second-order recursionthat is, person A may know not only what person B is thinking, but also that B knows what A is thinking. Indeed human social understanding may proceed well beyond the second level. Premack (2007, p. 13865) gives these examples:
John thinks that Bill thinks that Henry believes that John should put his kids in Sunday school.
Women think that men think that they think that men think that womens orgasm is different.
MIRROR NEURONS
Great apes almost certainly do not achieve an understanding of the minds of others at anything approaching this level of recursion, but the roots of theory of mind may go back to so-called mirror neurons in the primate brain. Mirror neurons, first recorded in area F5 of the monkey cortex (Di Pellegrino et al., 1992), are activated when the animal makes a movement to grasp an object, but are also activated when the monkey observes another individual making the same movement. Mirror neurons are now considered part of a more extensive mirror system, involving regions in the ventral prefrontal cortex, parietal cortex, and superior temporal sulcus (Figure 3; see Rizzolatti and Sinigaglia, 2010 for review). It is of interest that this system also overlaps extensively with the default network.
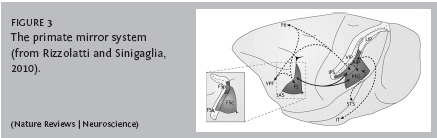
Because the mirror system maps the actions of others onto the actions of the observer, it may seem to provide a natural mechanism for the understanding of those actions. This was at first rejected on the grounds that monkeys dont imitate (Rizzolatti and Craighero, 2004), but more detailed investigation suggests that monkeys (Voelkl and Huber, 2007), along with other nonhuman animals, even budgerigars (Mui et al., 2008), can indeed imitate, albeit in fairly restricted ways. The role of the mirror system even in human imitation is controversial (see Gallese et al., 2011), although there is some evidence that failure of the mirror system plays a role in autism, which is thought to reflect defective theory of mind (e.g., Oberman, Ramachandran and Pineda, 2008). Be that as it may, it seems likely that mirror neurons in primates set the stage for the subsequent evolution of more complex mapping of the thoughts and actions of others onto ones own. In Galleses (2007) felicitous phrase, mirror neurons may be before and below theory of mind.
LANGUAGE
Although the default network may derive from structures present in the primate brain, and was therefore probably established well before the emergence of Homo sapiens or even the great apes, the general consensus seems to be that language evolved much more recently. As noted earlier, Chomsky and others have argued that language emerged anew, and with wholly novel properties, in our own species. This view was modified somewhat in an article co-authored by Chomsky (Hauser, Chomsky and Fitch, 2002), who distinguished between the faculty of language in the broad sense (FLB) and the faculty of language in the narrow sense (FLN). FLB includes necessary components of communication shared with other species, such as memory, circulation, respiration, perception, and the like. In humans only, it also includes FLN. In Chomskyan terms, FLN can be identified with what he has termed I-languagethe internal mode of thought that can be mapped into E-languagesthe external languages we actually (or potentially) speak or sign. According to Hauser et al. (2002), the critical feature of FLN is recursion.
Chomskys view is that I-language emerged in that single Promethean step that created unbounded Merge, and so established a new form of thought and language unique to our species. In some respects this view has its origins in the so-called language-of- thought (LOT) hypothesis elaborated by Fodor (1975). According to this view, human thought is a symbol-processing system like that of a digital computer, with a combinatorial syntax. Even mental imagery is assumed to be propositional, or language-like, rather than picture-like (e.g., Pylyshyn, 1973). Penn, Holyoak, and Povinelli (2008) suggest that symbolic representation was a uniquely human way of representing, or reinterpreting, the world:
Our most important claim [ ] is simply that whatever good trick [ ] was responsible for the advent of human beings ability to reinterpret the world in a symbolic-relational fashion, it evolved in only one lineageours. Nonhuman animals didnt (and still dont) get it [Penn, Holyoak and Povinelli, 2008, p. 129].
I argue here that language and symbolic representation emerged not so much as aspects of thought itself, but rather as a means for the sharing of thoughts between individuals. The two aspects of the default network described above, namely mental time travel and theory of mind, were probably necessary antecedents to the evolution of language itself (see also Corballis, 2011)and indeed the language circuit in the brain is also contained within the default network (see Figure 4). This approach allows a more continuous, Darwinian, approach to the evolution of language and thought than implied by modern Cartesians such as Chomsky and Penn et al.

LANGUAGE AND MENTAL TIME TRAVEL
If mental time travel is adaptive in that it facilitates planning and the understanding of the world, the sharing of our mental time travels is even more so. We often live vicariously through the exploits of others. Language is exquisitely tailored to allow this sharing, by enabling the communication of events that are not present in the here-and-now. Critical to both language and mental time travel are internal representations of the non-present. To even think about what happened yesterday or what might happen tomorrow, we need representations of the various actors, actions, and objects in the absence of their physical presence. Those representations might be pictorial or analogue in format, or they might assume more abstract properties. In order to communicate with others about them, we need of course to map them onto an output system, such as spoken words or manual signs. Sign languages maintain a degree of pictorialityor iconicityin representation, but tend to become more abstract over time. Spoken words are of course much less amenable to pictorial representation, although they can mimic acoustic information to some degree, as in words like crackle or tweet, or zanzara (the Italian word for mosquito).
Abstract representation is not so much a necessary property of language itself, as traditionally held (e.g., Saussure, 1916), but is more a matter of expediency, especially in the case of spoken language. Hockett (1978) put it like this:
[ ] when a representation of some four-dimensional hunk of life has to be compressed into the single dimension of speech, most iconicity is necessarily squeezed out. In one-dimensional projections, an elephant is indistinguishable from a woodshed. Speech perforce is largely arbitrary, if we speakers take pride in that, it is because in 50,000 years or so of talking we have learned to make a virtue of necessity [Hockett, 1978, pp. 274-275].
The process of increasing abstraction depends on what has been termed conventionalization, whereby conventions are established as to the meanings of symbols (Burling, 1999). It also occurs in sign languages, presumably because pictorial representations are often too elaborate, and are replaced by simpler and more abstract ones (Frishberg, 1975).
The ability to attach arbitrary symbols to objects and actions is not unique to humans, but has been abundantly demonstrated in other species. Perhaps the most famous is the bonobo Kanzi, who uses a keyboard of hundreds of nonrepresentational symbols to refer to objects and actions (Savage--Rumbaugh, Shanker and Taylor, 1998). Equally impressive, but perhaps less well known, are the linguistic and cognitive exploits, based on manual signs, of two captive gorillas, Koko and Michael (Patterson and Gordon, 2001), who are also said to use hundreds of signs . These apes also appear to understand many spoken English words, although they cannot produce them. This ability is not restricted to apes: A border collie called Rico has been shown to rapidly acquire the meanings of some 200 spoken English words (Kaminsky, Call, and Fischer, 2004). These animals also use these symbols to refer to nonpresent objects or actions, as when a chimpanzee uses a symbol to request a banana or asks to be tickled or to play, or when Rico goes to another room to fetch a designated object.
Of course we humans have much larger vocabularies of concepts, and words to name them. Pinker (2007) has estimated that the average literate person today knows some 50,000 concepts. This is based on the number of words in a college dictionary, implying that each concept is matched with a word. But where humans truly diverge is in the combinations of symbols. That is, where human language assumes a complexity greater than animal communication is not only in terms of the number of concepts and symbols, but also in the manner in which they combine to form meaningful combinations. At the level of thought, the combinations that humans can construct presumably far exceed those of the nonhuman wandering mind, perhaps driven by the complexities of mental time travel. And those complexities may have driven the complexities of syntax, as language is mapped onto remembered and planned episodes.
Syntax, then, may derive from the combinatorial nature of episodes. This too involves conventions. One such convention has to do with the ordering of symbols. For example, in communicating a scene such as The cow jumped over the moon, we need symbols for cow, jump, and moon. In signed languages, these symbols could be partly iconic; for example, the sign for jump could be conveyed in a semicircular movement, and that for moon as a circle shaped by the thumb and forefinger. In spoken language, of course, the symbols lose their iconic qualityalthough it might be noted that the word moon is uttered with the mouth shaped in circular fashion. In describing the scene as a whole, we need a convention as to the order. In some languages, such as English, the order is subject-verb-object (SVO), but there is considerable variation. It has been recently claimed that the original spoken language, assumed to have emerged in Africa prior to the migrations of Homo sapiens, used the order subject-object-verb (SVO), as in most Australian languages (Gell-Mann and Ruhlen, 2011). Some languages use other conventions to distinguish subject, object, and verb, and order assumes little or no importance.
We also need conventions to locate events in time; the suffix –ed in the word jumped, for example, indicates that the unlikely feat performed by the cow occurred in the past. Again, the definite article the signals a specific cow and a specific moon, and not just any old cow and moon. These conventions, like the symbols themselves, vary considerably between languages. In American Sign Language, for instance, a notional time line runs from behind the body to the front of the body. By doing a sign farther back you can indicate that the signed event occurred further in the past, while the farther forward an event is signed the further in the future it is. Signing close to the body can be used to indicate the nearness or recentness of an event.
Just why languages vary so much is not altogether clear. It has been estimated that there are at least 6,000 languages throughout the world, and their sheer variety casts doubt on the Chomskyan notion that all languages conform to a single underlying universal grammar (or I-language) (Evans, 2009). The rapid mutation of languages into different forms may have led to social processes enabling contact within groups but preventing those outside the group from intruding. Languages probably also conform to differing social needs. For instance, Everett (2005) has claimed that the Pirahã, a small community on the Amazon, have little concept of time, and presumably little need to represent the past or future, and their language correspondingly lacks tense, with the simple exception of a distinction between present and not present. Everett (in press) suggests that language is effectively a social tool, rather than an innate disposition, and is adapted to meet social and cultural needs.
It is largely through language, of course, that we share imaginary episodes, as in fiction. Episodes, whether based on the remembered past, the anticipated future, or sheer fantasy, can also be conveyed through pantomime and dance, and it may well be that language itself evolved from the gradual conventionalization of pantomime. Over time, other devices have been developed to relay information about episodes, whether real or imaginarythese include painting, photography, moving films, television, and YouTube. Of course language itself is so much a part of human episodes that it too plays a prominent role in those episodes themselves. In telling stories, we need to distinguish the voice of the teller from the voices of the characters depicted.
LANGUAGE AND THEORY OF MIND
The importance of theory of mind to language can be credited to Grice (1975), who held that language depends on inference rather than explicit decoding. Animal communication, like computer languages, is generally unambiguous, whereas human language, despite its apparent richness, is characteristically ambiguous and imprecise. In order to converse, individuals must understand what is going on in each others minds, so that each can infer what the other means. Chomsky (2007) expresses much the same idea:
Communication is not a matter of producing some mind-external entity that the hearer picks out of the world, the way a natural scientist could. Rather, communication is a more-or-less affair, in which the speaker produces external events and hearers seek to match them as best they can to their own internal resources. Words and concepts appear to be similar in this regard, even the simplest of them. Communication relies on largely shared cognoscitive powers, and succeeds insofar as similar mental constructs, background, concerns, and presuppositions allow for similar perspectives to be reached [Chomsky, 2007, p. 10].
As an example of the ambiguity of language, Sperber and Origgi (2010) give the sentence It was too slow. This could mean anything from a chemical reaction being too slow, to the decrease in unemployment in France being too slow, to a car being too slow for an anticipated journeyor a sluggish movement in a symphonic production. In uttering such a sentence, the speaker knows what is in the listeners mind, and has no need to elaborate further. She also knows that the listener knows whats in her mind. In this sense, conversational language, at least, serves as a series of prompts to guide shared thought.
The various unspoken thought processes and intentions underlying conversations are known as implicatures, and the manner in which speakers and listeners determine implicatures is one of the goals of the branch of linguistics known as pragmatics. Sperber and Wilson (2002) have argued that the decoding of implicatures depends on a specialized theory-of-mind module, in the sense proposed by Fodor (1983). Modules are assumed to be innate, and to operate automatically, so one might suppose that they carry out operations of a complexity similar to those that, say, allow us to maintain balance while walking. Even so, simply declaring a function to depend on an innate module does not tell us how it actually works.
Sperber and Wilson further suggest that there are sub-modules which help narrow down the alternative meanings, and so reduce the computational demand. For instance, we have a built-in sensitivity to where others are looking, and this can establish a common focus of attention. A statement such as Thats really weird can then be quickly understood to refer to any object at that focus. More generally, Sperber and Wilson (1986) suggest that we continually maximize the relevance of available inputs, whether from the outside world or from memory, which can include knowledge of the memories and cultural habits of the person we are conversing with. This immediately narrows the possible interpretation of utterances, and may allow a conversation to proceed with minimal explications.
We also calibrate the degree of specificity depending on the audience and the topic. That is, we need to be as sensitive to what is not in the mind of the listener as well as what is in her mind. Technical or legal language requires more specification to avoid ambiguity, and in lecturing to a first-year university class one assumes that much of what one says is novel to the listenersotherwise there would be no point in giving the lecture! It is perhaps doubtful whether language can achieve complete specification, as I know from attempts to construct an object such as a kitset trolley from written instructions. The course of a home-built trolley, like that of true love, never did run smooth.
Theory of mind also enables us to mentally travel into the minds of others, and tell stories from the perspectives of other individuals, whether real or imaginary. This is widely used in fiction, whether the narrator is or is not identified with the author. It is the vast capacity to wander mentally away from the present, as well as into the minds of others, along with the capacity to transmit our wanderings to others, that perhaps most clearly distinguishes the human mind from the animal mind. Even so, I think it unlikely that this was a sudden occurrence. Elements of it, albeit in reduced form, can be discerned in other species. The next question, then, is when did this enlarged capacity evolve?
WANDERING THROUGH THE PLEISTOCENE
If there was a leap forward, it probably took place largely during the Pleistocenea stretch of over two million years rather than an instantaneous event that occurred a mere 70,000 or so years ago. And it was perhaps not so much a leap as a quickening of pace.
Why the Pleistocene? As mentioned earlier, this era has been regarded by evolutionary psychologists as the EEA for human cognition. It saw the emergence of the new genus, Homo, of which Homo sapiens is the only extant member. Traditionally, early Homo has been dated to around 2.6 million years ago in the forms of Homo habilis and Homo rudolfensis, both associated with early stone tools, although it has been argued that both should really be classified as australopithecines rather than Homo (Wood and Collard, 1999). The recent discovery of a fossil identified as Australopithecus sediba, dated at just under 2 million years ago, perhaps lies more securely at the transition between Australopithecus and Homo, with a brain size in the australopithecine range but Homo-like hands (Kivell et al., 2011). Thereafter, brain size in the genus Homo increased approximately threefold, reaching a peak of 1515 cm3 in Neanderthals perhaps 300,000 years ago. The brain size of H. sapiens is slightly less, at an average of 1355 cm3. When brain size is expressed as a ratio of orbital size (a proxy for body size), sapiens emerges as marginally in advance of Neanderthals (Wood and Collard, 1999). Nevertheless these figures are scarcely indicative of a great leap forward unique to our species, but instead suggest gradual increases in cognitive capacity through the Pleistocene.
The increase in brain size is perhaps the clearest indication that human cognition advanced during the Pleistocene. A further clue is the emergence of stone tools, beginning with the Oldowan industry, associated with Homo habilis from around 2.3 million years ago, followed by the more complex Acheulian industry associated with Homo ergaster and Homo erectus from around 1.9 to 1.4 million years ago (Plummer, 2004). Gärdenfors and Osvath (2010) describe the Oldowan as a long ranging culture, characterized by an extension in time and spacethe emergence, in other words, of mental time travel. The Oldowan people ranged over large distances to gain raw materials or to scavenge or slaughter for food, and long time intervals intervened between the manufacture and use of tools. Gärdenfors and Osvath suggest that this heightened the reliance on prospective cognition, which they consider the basis for the subsequent emergence of symbolic communication.
More generally, the Pleistocene has been suggested as the era in which hominins came to occupy what has been termed the cognitive niche (Tooby and DeVore, 1987), depending for survival in the more exposed and dangerous environment on social bonding and enhanced communication. The cognitive niche included elaboration of theory of mind, in the emergence of what has also been called the social mind as it evolved during the Pleistocene (e.g., Forgas, Haselton and von Hippel, 2007). It should not be thought, though, that the social mind evolved de novo in the genus Homo. All primates are social creatures, and even in macaques increasing the size of the social network results in increases in gray matter in prefrontal cortex and superior temporal lobe (Sallet et al., 2011)areas homologous with the default network in humans. According to one analysis, social living co-evolved with a change from nocturnal to diurnal activity, driven perhaps by greater danger of predation during daytime hours and the protections afforded by social bonding. Sociality, it is suggested, progressed from solitary foraging individuals to large multi-male/multi-female aggregations approximately 52 million years ago, with pair-living or single-male harem systems emerging about 16 million years ago (Shultz, Opie, and Atkinson, 2011). These developments may have set the stage for the more elaborate social structures that emerged in our hominin forebears, especially during the Pleistocene.
At what point, then, did language itself emerge? One possibility, of course, is that language did indeed emerge suddenly in a great leap forward within the past 100,000 years, as proposed by Chomsky (2010) and others. As noted earlier, this is difficult to reconcile with the Darwinian theory of natural selection. From an evolutionary perspective, the more plausible scenario is that the steps leading to language were incremental. This need not mean, though, that language was simply a series of small adaptations, each leading to an incremental increase in language capacity. Many of the changes were probably better described as exaptations, derived from structures that had evolved for other reasons. For example the recursive structure of language may derive from theory of mind, which evolved increasingly complex recursive loops of mind reading and deceptionas Machiavelli (1961 [1532]) himself put it It is double pleasure to deceive the deceiver. Recursive grammar may therefore be an exaptation from recursive thought, or it may have coevolved with it.
DID LANGUAGE EVOLVE FROM MANUAL GESTURES?
A growing possibility consistent with gradual evolution is the view that language evolved from manual gestures. This argument has been detailed elsewhere (e.g., Arbib, 2005; Armstrong, 1999; Corballis, 2002, 2009; Rizzolatti and Sinigaglia, 2008; Tomasello, 2008). Briefly, language is assumed to have ancient roots in the primate mirror system, which provided the platform for the production and learning of manual actions, and was initially specialized for manual grasping. This system was extended to communicative acts in great apes, and to the pantomiming of action episodes in hominins. The sophistication of bodily pantomime may well have been enhanced by bipedalism. The progression toward more complex and conventionalized description of episodes may well have occurred during the Pleistocene, culminating in a generative form of communication that we can identify as language. In late Homo, including the Neanderthals, language may have remained predominantly manual, although perhaps with increasing facial and vocal elements.
The critical event that led to the apparent human revolution of the past 100,000 years of our own species may then have depended, not on the emergence of language itself, but rather on a final conversion to speech as the dominant mode. Although the transition from a manual to a vocal system may seem extreme, it is likely that the incorporation of speech sounds was gradual (Gentilucci and Corballis, 2006), and even today manual gesture forms an integral part of everyday speech (Kendon, 2004; McNeill, 1985), and shares the same neurological substrate (Bernardis and Gentilucci, 2006). Indeed, manual gesture precedes speech in human development (Volterra, et al., 2005), perhaps recapitulating the evolutionary sequence. Moreover, speech itself can be regarded as a form of gesture, involving movements of six articulatory organs, the lips, the velum, the larynx, and the blade, body, and root of the tongue (Studdert-Kennedy, 2005). Because these organs are largely hidden from view, adding sound was necessary to make them accessible. Speech, then, is noisy gesture. The retreat of language from extensive bodily movements to the mouth was perhaps an early example of miniaturization, freeing the rest of the body for other actions. This increase in bodily freedom may have been the main source of the explosion of manufacture and art that made up the great leap forward as identified by some archeologists, or the emergence of what has been described as modernity (Corballis, 2004).
The emergence of a predominantly vocal form of communication may have been driven by other advantages as well. Manual language requires light, whereas speech can be conducted at night, in darkness, or when obstacles prevent visual access. Manual language is effortful, requiring considerable expenditure of energy, while the physiological costs of speech are so low as to be nearly unmeasurable (Russell, Cerny and Stathopoulos, 1998). Speech adds little to the cost of breathing, which we must do anyway to sustain life. Speech is probably more attention-gettingno amount of gesture will awaken members of an audience who have gone to sleep. It should be emphasized that the advantages of speech are practical, rather than linguistic, since it is widely acknowledged that signed languages possess all of the grammatical and semantic complexity of speech (e.g., Emmorey, 2002; Neidle et al., 2000), and American Sign Language is the language of instruction at Gallaudet University in Washington, DC. Of course there are even situations in which the practical advantages lie with signing, as in hunting where voicing may betray position, or in extremely noisy environments.
The evolutionary progression implied by the gestural theory should again be understood in terms of exaptations as well as adaptations, but nonetheless incremental and driven by natural selection. Manual gestures were exapted from manual grasping, the transition from manual to facial gesture were exapted from pre-existing links perhaps associated with eating, the production of speech was exapted from mechanisms specialized for eating, breathing, and of course involuntary animal calls. The transitions from animal behavior to human thought and language are no doubt more complex and intertwined than can be spelled out in detail here, but the challenge is to understand them without appeal to miracles.
CONCLUSIONS
The human mind has evolved to wander, not only back and forth in time, but also into imaginary worlds, and into the minds of others. The extent to which this facility occurs in other species remains unknown, although it seems likely that the mind-wandering of humans is considerably more flexible than that of even our closest nonhuman relatives, chimpanzees and bonobos. One reason to suppose that this is so is that language appears to have evolved to allow us to share our mind-wanderings, and there is so far little evidence that anything resembling language exists in other speciesat least with respect to its generativity and infinite range of expression. It is of course conceivable that chimpanzees have rich mental lives but lack the means to express their mental wanderings.
The neurological underpinnings of mind wandering is the default network, identified largely through brain imaging in humans, but seemingly anatomically present in monkeys (Vincent et al., 2007). It is likely that primates have at least some degree of internal processing, independent of external input, but that the default network underwent considerable elaboration in our Homo ancestors. The Pleistocene, which saw the emergence of the genus Homo from earlier ape-like australopithecines, marked a dramatic change in ecology, with a succession of ice ages and the loss of protective forest canopy, forcing our forebears onto the relatively open savanna. An especially dangerous feature of the savanna was the presence of large carnivorous animals, whose numbers peaked in the early Pleistocene. They included at least twelve species of saber-tooth cats and nine species of hyena (Foley, 1984). Our forebears had previously been able to seek cover from these dangerous predators in more forested areas, and perhaps by retreating into water, but such means of escape were relatively sparse on the savanna. Not only did the hominins have to avoid being hunted down by these formidable killers, with their sharp teeth and claws, and immense speed and strength, but they also had to compete with them for food resources.
It was perhaps these conditions that led to enhanced social cognition, involving extensive mind-sharing and capacity for social planning. The changes were dramatic, as is especially clear from the seemingly unique nature of human language, and perhaps also from the human capacity to think recursively (Corballis, 2011). But we need not assume that these changes came about suddenly, through a single mutation late in the prehistory of our own species. There is sufficient evidence from the limited ability of nonhuman primates to socialize, to remember, plan, and communicate through gestures, as well as developments such as increased brain size and cultural innovations during the relatively broad time window of the Pleistocene, to support the idea that our distinctively human mind was formed through natural selection.
REFERENCES
ADDIS, D.R., WONG, A.T., SCHACTER, D.L. (2007), Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 45, pp. 1363-1377. [ Links ]
ARBIB, M.A. (2005), From monkey-like action recognition to human language: An evolutionary framework for neurolinguistics. Behavioral and Brain Sciences, 28, pp. 105-168. [ Links ]
ARMSTRONG, D.F. (1999), Original signs: Gesture, Sign, and the Source of Language, Washington, DC, Gallaudet University Press. [ Links ]
BAR, M. (2009), The proactive brain: Memory for predictions. Philosophical Transactions of the Royal Society B, 364, pp. 1235-1243. [ Links ]
BERNARDIS, P., GENTILUCCI, M. (2006), Speech and gesture share the same communication system. Neuropsychologia, 44, pp. 178-190. [ Links ]
BOYD, B. (2009), The Origin of Stories: Evolution, Cognition, and Fiction, Cambridge, MA, Belnap Press of Harvard University Press. [ Links ]
BUCKNER, R.L., ANDREWS-HANNA, J.R., SCHACTER, D.L. (2008), The brains default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, pp. 1-38. [ Links ]
BUCKNER, R.L., VINCENT, J.L. (2007), Unrest at rest: default activity and spontaneous network correlations. Neuroimage, 37, pp. 1091-1096. [ Links ]
BURLING, R. (1999), Motivation, conventionalization, and arbitrariness in the origin of language. In B.J. King (ed.), The Origins of Language: What Nonhuman Primates Can Tell Us, Washington, DC, School of American Research Press, pp. 307-350. [ Links ]
CALL, J., TOMASELLO, M. (2008), Does the chimpanzee have a theory of mind? 30 years later. Trends in Cognitive Sciences, 12, pp. 187-192. [ Links ]
CHOMSKY, N. (2007), Biolinguistic explorations: Design, development, evolution. International Journal of Philosophical Studies, 15, pp. 1-21. [ Links ]
CHOMSKY, N. (2010), Some simple evo devo theses: How true might they be for language? In R.K. Larson, V. Déprez and H. Yamakido (eds), The Evolution of Human Language, Cambridge, Cambridge University Press, pp. 45-62. [ Links ]
CLAYTON, N.S., BUSSEY, T.J., DICKINSON, A. (2003), Can animals recall the past and plan the future?. Nature Reviews Neuroscience, 4, pp. 685-690. [ Links ]
CORBALLIS, M.C. (2002), From Hand to Mouth: the Origins of Language, Princeton, NJ, Princeton University Press. [ Links ]
CORBALLIS, M.C. (2004), The origins of modernity: Was autonomous speech the critical factor?. Psychological Review, 111, pp. 543-522. [ Links ]
CORBALLIS, M.C. (2009), The evolution of language. Proceedings of the New York Academy of Sciences, 1156, pp. 19-43. [ Links ]
CORBALLIS, M.C. (2011), The Recursive Mind: The Origins of Human Language, Thought, and Civilization, Princeton, NJ, Princeton University Press. [ Links ]
CORBALLIS, M.C. (2013), Mental time travel: A case for evolutionary continuity. Trends in Cognitive Sciences (in press). [ Links ]
CORREIA, S.P.C., DICKINSON, A., CLAYTON, N.S. (2007), Western scrub-jays anticipate future needs independently of their current motivational state. Current Biology, 17, pp. 856-861. [ Links ]
DARWIN, C. (1859), On the Origin of Species, London, John Murray. [ Links ]
DI PELLEGRINO, G., et al. (1992), Understanding motor events: A neurophysiological study. Experimental Brain Research, 91, pp. 176-180. [ Links ]
EMMOREY, K. (2002), Language, Cognition, and Brain: Insights from sign Language Research, Hillsdale, NJ, Erlbaum. [ Links ]
EVANS, N. (2009), Dying words: Endangered Languages and What They Have to Tell Us. Oxford, Wiley-Blackwell. [ Links ]
EVERETT, D.L. (2005), Cultural constraints on grammar and cognition in Pirahã. Current Anthropology, 46, pp. 621-646. [ Links ]
EVERETT, D.L. (in press), Cognitive Fire: Language as a Cultural Tool, New York, Pantheon Books. [ Links ]
FODOR, J.A. (1975), The Language of Thought, Cambridge, MA, Harvard University Press. [ Links ]
FODOR, J.A. (1983), The Modularity of Mind, Cambridge, MA, Bradford Books, MIT Press. [ Links ]
FOLEY, R. (1984), Early man and the Red Queen: Tropical African community evolution and hominid adaptation. In R. Foley (ed.), Hominid Evolution and Community Ecology: Prehistoric Human Adaptation in Biological Perspective, London, Academic Press, pp. 85-110. [ Links ]
FORGAS, J.P., HASELTON, M.G., VON HIPPEL, W. (2007), Evolution and the Social Mind: Evolutionary Psychology and Social Cognition, London, Psychology Press. [ Links ]
FRISHBERG, N. (1975), Arbitrariness and iconicity in American Sign Language. Language, 51, pp. 696-719. [ Links ]
GALLESE, V. (2007), Before and below theory of mind: embodied simulation and the neural correlates of social cognition. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 362, pp. 659-669. [ Links ]
GALLESE, V., et al. (2011), Mirror neuron forum. Perspectives on Psychological Science, 6, pp. 369-407. [ Links ]
GÄRDENFORS, P., OSVATH, M. (2010), Prospection as a cognitive precursor to symbolic communication. In R.K. Larson, V. Déprez, H. Yamakido (eds.), The Evolution of Human Language, Cambridge, Cambridge University Press, pp. 103-114. [ Links ]
GELL-MANN, M., RUHLEN, M. (2011), The origin and evolution of word order. Proceedings of the National Academy of Sciences, 108, pp. 17290-17295. [ Links ]
GENTILUCCI, M., CORBALLIS, M.C. (2006), From manual gesture to speech: A gradual transition. Neuroscience and Biobehavioral Reviews, 30, pp. 949-960. [ Links ]
GRICE, H.P. (1975), Logic and conversation. In P. Cole, J. Morgan (eds.), Syntax and Semantics, vol. 3, Speech acts, New York, Academic Press, pp. 43-58. [ Links ]
GUPTA, A.S., et al. (2010), Hippocampal replay is not a simple function of experience. Neuron, 65, pp. 695-705. [ Links ]
HASSABIS, D., KUMARAN, D., MAGUIRE, E.A. (2007), Using imagination to understand the neural basis of episodic memory. Journal of Neuroscience, 27, pp. 14365-14374. [ Links ]
HAUSER, M.D., CHOMSKY, N., FITCH, W.T. (2002), The faculty of language: What is it, who has it, and how did it evolve?. Science, 298, pp. 1569-1579. [ Links ]
HOCKETT, C.F. (1978), In search of loves brow. American Speech, 53, pp. 243-315. [ Links ]
HOFFECKER, J.F. (2007), Representation and recursion in the archaeological record. Journal of Archaeological Method and Theory, 14, pp. 359-387. [ Links ]
KAMINSKY, J., CALL, J., FISCHER, J. (2004), Word learning in a domestic dog: Evidence for fast-mapping. Science, 304, pp. 1682-1683. [ Links ]
KENDON, A. (2004), Gesture: Visible Action as Utterance, Cambridge, Cambridge University Press. [ Links ]
KIVELL, T.L., et al. (2011), Australopithecus sediba demonstrates mosaic evolution of locomotor and manipulative abilities. Science, 333, pp. 1411-1417. [ Links ]
KLEIN, R.G. (2008), Out of Africa and the evolution of human behaviour. Evolutionary Anthropology, 17, pp. 267-281. [ Links ]
KOVÁCS, A.M., TÉGLÁS, E., ENDRESS, A.D. (2010), The social sense: Susceptibility to others beliefs in human infants and adults. Science, 330, pp. 1830-1834. [ Links ]
MACCHIAVELLI, N. (1961 [1532]), The Prince, London, Penguin (translated by George Bull). [ Links ]
McBREARTY, S., BROOKS, A.S. (2000), The revolution that wasnt: A new interpretation of the origin of modern human behavior. Journal of Human Evolution, 39, pp. 453-563. [ Links ]
McNEILL, D. (1985), So you think gestures are nonverbal?. Psychological Review, 92, pp. 350-371. [ Links ]
MELLARS, P.A. (2006), Going east: New genetic and archaeological perspectives on the modern colonization of Eurasia. Science, 313, pp. 796-800. [ Links ]
MELLARS, P.A., STRINGER, C.B. (eds) (1989), The Human Revolution: Behavioral and Biological Perspectives on the Origins of Modern Humans, Edinburgh, Edinburgh University Press. [ Links ]
MUI, R., et al. (2008), Automatic imitation in budgerigars. Proceedings of the Royal Society of London B: Biological Sciences, 275, pp. 2547-2553. [ Links ]
NEIDLE, C., et al. (2000), The Syntax of American Sign Language, Cambridge, MA, The MIT Press. [ Links ]
OBERMAN, L.M., RAMACHANDRAN, V.S., PINEDA, J.A. (2008), Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia, 46, pp. 1558-1565. [ Links ]
OSVATH, M. (2011), Spontaneous planning for future stone throwing by a male chimpanzee. Current Biology, 19, R190. [ Links ]
OSVATH, M., OSVATH, H. (2008), Chimpanzee (Pan troglodytes) and orangutan (Pongo abelii) forethought: self-control and pre-experience in the face of future tool use. Animal Cognition, 11, pp. 661-674. [ Links ]
PATTERSON, F.G.P., GORDON, W. (2001), Twenty-seven years of project Koko and Michael. In B.M.F. Galdikas et al., All Apes Great and Small, African Apes , vol. 1, New York, Kluver, pp. 165-176. [ Links ]
PENN, D.C., HOLYOAK, K.J., POVINELLI, D.J. (2008), Darwins mistake: Explaining the discontinuity between human and nonhuman minds. Behavioral and Brain Sciences, 31, pp. 108-178. [ Links ]
PENN, D.C., POVINELLI, D.J. (2007), On the lack of evidence that non-human animals possess anything remotely resembling a theory of mind. Philosophical Transactions of the Royal Society B, 362, pp. 731-744. [ Links ]
PINKER, S. (2007), The Stuff of Thought, London, Penguin Books. [ Links ]
PLUMMER, T. (2004), Flaked stones and old bones: Biological and cultural evolution at the dawn of technology. Yearbook of Physical Anthropology, 47, pp. 118-164. [ Links ]
PREMACK, D. (2007), Human and animal cognition: Continuity and discontinuity. Proceedings of the National Academy of Sciences (USA), 104, pp. 13861-13867. [ Links ]
PREMACK, D., WOODRUFF, G. (1978), Does the chimpanzee have a theory of mind?. Behavioral and Brain Sciences, 4, pp. 515-526. [ Links ]
PYLYSHYN, Z.W. (1973), What the minds eye tells the minds brain: A critique of mental imagery. Psychological Bulletin, 80, pp. 1-24. [ Links ]
RAICHLE, M.E., et al. (2001), A default mode of brain function. Proceedings of the National Academy of Sciences, 109, pp. 3979-3984. [ Links ]
RIZZOLATTI, G., CRAIGHERO, L. (2004), The mirror-neuron system. Annual Review of Neuroscience, 27, pp. 169-192. [ Links ]
RIZZOLATTI, G., SINIGAGLIA, C. (2008), Mirrors in the Brain, Oxford, Oxford University Press. [ Links ]
RIZZOLATTI, G., SINIGAGLIA, C. (2010), The functional role of the fronto-parietal mirror circuit: Interpretations and misinterpretations. Nature Reviews Neuroscience, 11, pp. 264-274. [ Links ]
RUSSELL, B.A., CERNY, F.J., STATHOPOULOS, E.T. (1998), Effects of varied vocal intensity on ventilation and energy expenditure in women and men. Journal of Speech, Language, and Hearing Research, 41, pp. 239-248. [ Links ]
SALLET, J., et al. (2011), Social network size affects neural circuits in macaques. Science, 334, pp. 697-700. [ Links ]
SAUSSURE, F. de (1916), Cours de linguistique générale, ed. C. Bally and A. Sechehaye, translated as W. Baskin (1977), Course in General Linguistics, London, Fontana/Collins. [ Links ]
SAVAGE-RUMBAUGH, S., SHANKER, S.G., TAYLOR, T.J. (1998), Apes, Language, and the Human Mind, Oxford, Oxford University Press. [ Links ]
SHULTZ, S., OPIE, C., ATKINSON, Q.D. (2011), Stepwise evolution of stable sociality in primates. Nature, 479, pp. 219-224. [ Links ]
SPERBER, D., ORIGGI, G. (2010), A pragmatic perspective on the evolution of language. In R.K. Larson, V. Déprez and H. Yamakido (eds.), The Evolution of Human Language, Cambridge, Cambridge University Press, pp. 124-131. [ Links ]
SPERBER, D., WILSON, D. (1986), Relevance: Communication and Cognition, Oxford, Blackwell. [ Links ]
SPERBER, D., WILSON, D. (2002), Pragmatics, modularity and mind-reading. Mind and Language, 17, pp. 3-23. [ Links ]
STUDDERT-KENNEDY, M. (2005), How did language go discrete? In M. Tallerman (ed.), Language Origins: Perspectives on Evolution, Oxford, UK, Oxford University Press, pp. 48-67. [ Links ]
SUDDENDORF, T., CORBALLIS, M.C. (1997), Mental time travel and the evolution of the human mind. Genetic, Social, and General Psychology Monographs, 123, pp. 133-167. [ Links ]
SUDDENDORF, T., CORBALLIS, M.C. (2007), The evolution of foresight: What is mental time travel, and is it unique to humans?. Behavioral and Brain Sciences, 30, pp. 299-351. [ Links ]
SZPUNAR, K.K., WATSON, J.M., McDERMOTT, K.B. (2007), Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences (USA), 104, pp. 642-647. [ Links ]
TOMASELLO, M. (2008), The Origins of Human Communication, Cambridge, MA, MIT Press. [ Links ]
TOOBY, J., COSMIDES, L. (1992), The psychological foundations of culture. In J. Barkow, L. Cosmides and J. Tooby (eds.), The Adapted Mind: Evolutionary Psychology and the Generation of Culture, New York, Oxford University Press, pp. 19-136. [ Links ]
TOOBY, J., DeVORE, I. (1987), The reconstruction of hominid behavioral evolution through strategic modeling. In W.G. Kinzey (ed.), The Evolution of Human Behavior: Primate models, Stony Brook, SUNY Press, pp. 183-237. [ Links ]
TULVING, E. (1972), Episodic and semantic memory. In E. Tulving and W. Donaldson (eds.), Organization of Memory, New York, Academic Press, pp. 381-403. [ Links ]
VINCENT, J.L., et al. (2007), Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 447, pp. 83-86. [ Links ]
VOELKL, B., HUBER, L. (2007), Imitation as faithful copying of a novel technique in marmoset monkeys. PLoS ONE, 2, e611. [ Links ]
VOLTERRA, V., et al. (2005), Gesture and the emergence and development of language . In M. Tomasello and D. Slobin (eds.), Beyond Nature-Nurture. Essays in Honor of Elizabeth Bates, Mahwah, NJ, Lawrence Erlbaum Associates, pp. 3-40. [ Links ]
WIMMER, H., PERNER, J. (1983), Beliefs about beliefs: Representation and constraining function of wrong beliefs in young childrens understanding of deception. Cognition, 13, pp. 103-128. [ Links ]
WOOD, B., COLLARD, M. (1999), The human genus. Science, 284, pp. 65-71. [ Links ]
Received 01-08-2012 . Accepted for publication 25-10-2012.














