Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Ciência e Técnica Vitivinícola
Print version ISSN 0254-0223
Ciência Téc. Vitiv. vol.27 no.1 Dois Portos 2012
Phenolic compounds released from oak, cherry, chestnut and robinia chips into a synthetic wine: influence of toasting level
Compostos fenólicos cedidos a um vinho sintético por aparas de carvalho, cerejeira, castanheiro e robinia: influência do nível de queima
Bruno Soares, Raquel Garcia, Ana Maria Costa Freitas, Maria João Cabrita*
1Instituto de Ciências Agrárias e Ambientais Mediterrânicas (ICAAM), Departamento de Fitotecnia, Escola de Ciências e Tecnologia, Universidade de Évora, Ap. 94 7002-554 Évora, Portugal.
SUMMARY
Advantages and drawbacks from the use of oak chips in oenology are well documented; wood oenological value is primarily related to cooperage potential, and not addressed from the oenologist view; for this reason other woods did not had similar attention. Polyphenols release is determinant to recognize the oenological value. Untoasted, light, medium and heavy toasted chips from oak, cherry, robinia and chestnut woods were add, to a model wine solution. Total polyphenolic content using Folin Ciocalteau reagent, absorbance at 280nm, identification and quantification of low molecular weight phenolic compounds were determined. Oak and chestnut released high amounts of phenolic compounds whilst cherry presented different behaviour. Robinia had the poorest performance, regarding low molecular weight phenolic compounds; the high values of absorbance and total polyphenolic found suggest the release of different phenolics. To our knowledge, this is the first study reporting toasting influence on the release of phenolic compounds from other kinds of wood species than oak.
Key words: oak, chestnut, robinia, cherry, toasting level, phenolic compounds.
RESUMO
As vantagens e as limitações do uso de aparas de carvalho em enologia encontram-se bem documentadas; o potencial que as madeiras apresentam para a tanoaria, tem sido mais importante que o seu impacto nos vinhos, e por isso outras espécies botânicas receberam menos atenção. Os polifenois que as madeiras cedem ao vinho são muito importantes do ponto de vista enológico. Aparas de carvalho, cerejeira, robinia e acácia com diferentes níveis de queima foram adicionadas a soluções hidroalcoólicas, e nelas foram determinados o teor em polifenois totais, a absorvancia a 280nm e os compostos fenólicos de baixo peso molecular. Os resultados mostram que o carvalho e o castanheiro libertam teores elevados de compostos fenólicos, ao contrário da cerejeira. Para a robinia, os resultados sugerem a libertação de outos compostos fenólicos para além dos de baixo peso molecular. De acordo com o nosso conhecimento, este é o primeiro trabalho que descreve a influência do nível de queima das aparas na libertação de compostos fenólicos, em outras espécies botânicas para além do carvalho.
Palavras-chave: carvalho, castanheiro, robinia, cerejeira, nível de queima, compostos fenólicos
INTRODUCTION
Wood has been used in alcoholic beverages for centuries, mainly as material for storage and by their role on the aging process. In 2005, OIV (Organization International de la Vigne et du Vin) approved the use of chips and staves (Resolution oeno 3/2005) as alternatives for barrels. However, this authorization is restricted to oak and chestnut wood species. The increased used of these alternatives are mainly related to low investments, similar sensorial results obtained in shorter time, simplicity of use and the possibility of avoiding contamination and off-flavours, too-often related to aged or contaminated barrels (Natali et al., 2006, Fernández de Simón et al., 2009).
Besides oak, other woods are being look at for oenological purposes, such as robinia, cherry, chestnut, mulberry, alder, ash, and beech (Madrera et al., 2010). Their characteristics are commonly compared to oak. In the past, chestnut (Castanea sativa Mill) was widely used in the Mediterranean area, because of its availability and its cheap price. Chestnut wood has higher porosity than oak. Cherry wood (Prunus avium L., Prunus cerasus L.) has a high porosity and oxygen permeation, and is usually use for short aging times. Robinia wood (Robinia pseudoacacia L.) is hard, with low porosity (Citron, 2005). In literature,Robinia pseudoacacia is often refer as acacia, but its proper name is robinia. However, the use of some type of wood species for cooperage was declined due to the lack of physic- chemical properties. Nowadays, the use of staves or chips opens different perspectives and the study of the oenological properties of several botanical species potentially useful for wine aging process should be restarted.
All woods are composed of cellulose, hemicellulose, lignin, ash-forming minerals, and extractives formed into a cellular structure. The characteristics and amounts of these compounds and differences in cellular structures give each wood its specific characteristics. Extractives, from different wood species, comprise several substances, which belong to an extremely wide range of chemical families, characteristics of each wood.
When heat is applied, during toasting process, chemical bonds are disrupted within biopolymers such as cellulose, hemicelluloses, lignin, polyphenols and lipids, resulting in degradation or compositional changes by pyrolisis and thermolysis (Fernández de Simón et al., 2009; Van Jaarsveld et al., 2009), which induce a notable modification on wood chemical composition. Phenolic aldehydes are mainly formed from lignin thermo degradation while degradation of polyosides leads to the production of furanic aldehydes.
The intensity and length of the applied heat in toasting process determine the toasting level, affecting, indubitably, the final wood chemical composition. Despite the scientific works made in order to optimize the heat treatment of the woods, designations like untoasted, light, medium and heavy toast are common, but there is no industry common standard for toast level.
The response of a wood to a particular seasoning and toasting conditions is also determined by size, as it affects their structural properties, and hence, the final flavour characteristics. Moreover, each type of piece size shows different extraction kinetics when in contact with wines (Fernández de Simón et al., 2010).
Aiming to evaluate the release of phenolic compounds from chips into simple model matrices, namely hydroalcoholic solutions, chips from four different wood species and with four levels of toasting were use, in order to compare the effect of botanical species (oak, robinia, cherry and chestnut) and the toasting level.
MATERIAL AND METHODS
Chemicals
The water employed was previously purified in a Mili-Q system (Millipore, Bedford, MA, USA). HPLC grade methanol and formic acid was purchase from Merck (Darmstadt, Germany). Extrasynthese (Genay, France) supplied gallic acid, protocatechuic aldehyde, syringic acid, and vanillic acid. Coniferaldehyde and sinapaldehyde were from Sigma-Aldrich (St Louis, MO). Furfural was from Merck (Darmstadt, Germany). 5-methylfurfural, 5-hydroxymethylfurfural, syringaldehyde and ellagic acid dehydrate were purchased from Acrós Organics (New Jersey, USA). 5-hydroxymethylfurfural was quantified as furfural.
Samples
Wood samples from robinia, chestnut, oak and cherry were provided as chips, with four different toasting levels: untoasted (UT), light toast (LT, 2 hours at 160ºC), medium toast (MT, 2 hours at 200ºC) and heavy toast (HT, 2 hours at 240ºC), by JM Gonçalves Cooperage industry. Woods were seasoned in the open air during 25 months for cherry and robinia, 22 month for chestnut and 32 month for oak. Botanical species were: oak (Quercus robur L.) chestnut (Castanea sativa Mill), robinia (unknown specie), and cherry (Prunus cerasus L.). Robinia is usually Robinia pseudoacacia L., false acacia or black locust, commonly and wrongly named acacia instead of robinia. Chestnut and oak woods are from North of Portugal, robinia and cherry woods are from Central France.
Wood extraction
Hydroalcoholic solutions (model wine) were prepared in order to have a final solution with 12% (v/v), ethanol, and a pH of 3,2 (tartarate buffer). Twelve grams of each type of wood, in the shape of chips, were extracted with 250 mL of the synthetic wine during 35 days, in the dark at room temperature, based on De Rosso et al. (2009). Extraction solutions were filtered through a nylon fi lter 0.45μm (Whatman, Schleicher & Schuell, England) and stored at 3ºC. Extractions were performed in duplicate.
HPLC Methods
For HPLC analysis, samples were filtered through a nylon fi lter 0.45μm (Whatman, Schleicher & Schuell, England), into a vial before injection. Analyses were performed in duplicate.
The HPLC system UltiMate 3000 (Dionex Corp., Sunnyvale, C.A.) comprises a quaternary pump, a column oven, a DAD detector and an autosampler. Chromeleon 7.0 software was used for acquisition and data treatment. The column was a LiChrosphere RP18, 5μm, 250x4mm (Merck, Darmstadt, Germany) and was kept isothermal, at 40ºC, during analysis. Eluent A was water: formic acid (98:2 (v/v)) and B was methanol: water: formic acid (70:28:2 (v/v/v)). The flow rate: 1 mLmin-1, injection volume was 25 μL and the elution program used was as follow: from 0% to 40% of solvent B in 45 minutes; 40% to 60% of solvent B in 25 minutes (Canas et al., 2003).
LC-DAD/ESI-MS method
Liquid Chromatography was performed using a Surveyor Thermo Finnigan HPLC system with autosampler and PDA detector. MS analysis was carried out in an LCQ Fleet mass spectrometer (Thermo Finnigan – San Jose, CA, USA) equipped with electrospray ionization (ESI) source and an ion trap mass analyzer. The column used was a Grace Smart RPC18, 150 mm × 2.1 mm ID, 3.0 μm. Mobile phase was a binary and consisted on solvents A (water- formic acid (99.9:0.1 (v/v)) and B (methanol) as follows: 5-45% B from 0-35 min; then 70% B from 35-40 min and 70% B from 40-50 min. The flow rate was fixed at 0.2mLmin-1 during the entire run. Column oven was kept isothermal at 30°C.Sample tray was set at 24 °C. Injection volume was 10 μL, and PDA detection was set between 200-600 nm to monitor the UV-Vis absorption. The conditions for MS analysis were: capillary temperature of 300°C, source voltage of 5.0 kV, source current of 100.0 μA and capillary voltage of -20.0 V in negative ion mode. Dry temperature was set to 300ºC and the flow of the drying gas as 50.0 arbitrary units.
Analytes were detected in full MS scan mode (m/z 100–1200). The source fragmentation impact was set at 30V whenever additional fragmentation was needed for compound identification.
The identification of chromatographic peaks was made by comparing their retention HPLC times with those of the external standards, as well as by ESI-MS and UV spectra analysis.
Spectrophotometric methods
For total polyphenols and spectrophotometric analyses, extracts were diluted 20 folds with deionised water and filtered. Total polyphenols were determined by the Folin-Ciocalteau method applied to wines (Singleton and Rossi, 1965). Absorbance spectra were recorded in the 250-450 nm range, with a 1 cm pathway cell against water, in a Cadas 100 spectrophotometer. All analysis was performed in duplicate.
Calibration curves
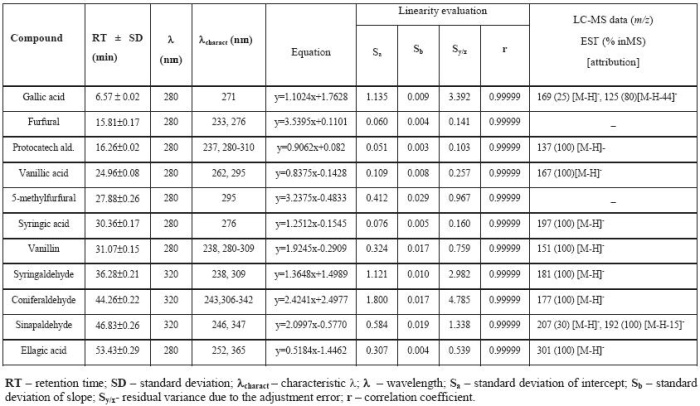
Standards were prepared in water except for elagic acid which was prepared in absolute ethanol. Calibration curves were obtained by injection of a series of standards diluted with a hydroalcoholic solution of 12% v/v, from a stock solution, according to the calibration data in Table I. All curves were obtained with at least six points. Injections were performed in duplicate.
Parameters for the calibration, spectroscopic and spectrometric data of low molecular weight phenolic compounds
Parâmetros de calibração, dados espectroscópicos e espectrofotométricos dos compostos fenólicos de baixo peso molecular
Statistical methods
Differences in the phenolic composition of wood chips were assessed by one-way analysis of variance (ANOVA), considering toasting level as a factor; mean comparisons were performed using Tukey-Kramer Multiple-Comparison Test at the 95% confidence level. Analyses were accomplished using NCSS 6.0 software (Statistical & Power Analysis Software, Kaysville, UT, USA).
RESULTS AND DISCUSSION
Figure 1 shows the absorbance spectra of the hydroalcoholic extracts, recorded from 250 to 450 nm. Chestnut and oak present a very similar spectra regardless toasting level, with a maximum absorption around 300nm, more highlighted with increasing toast level. Spectra from cherry and robinia present a maximum absorption at 280 nm, diminishing with toasting level. Cherry extracts are the ones with lower absorbances. Cherry and robinia spectra’s seem to indicate toasting influence on wood ability to release compounds into a hydroalcoholic solution.
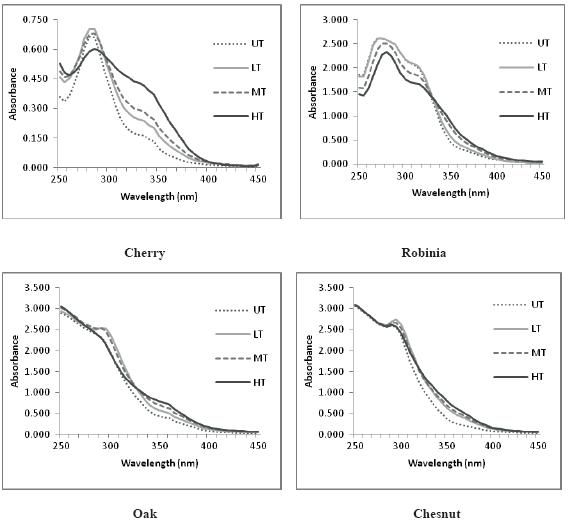
Figure 1 Absorbance spectra (250-450nm) of hydroalcoholic extracts of the four wood types subjected to different toasting levels. UT – untoasted; TL – light toast; MT – medium toast; HT – heavy toast
Espectros de absorvância (250-450nm) dos extratos hidroalcoólicos das quatro espécies sujeitas a diferentes níveis de queima. UT – não queimada; TL – queima ligeira; MT – queima média; HT – queima forte
Regarding total polyphenol contents of the four wood species hydroalcoholic extracts (Table II) it seems clear that chestnut and oak woods are the richest, while cherry is the poorest. This profi le is not influenced by toasting process. It was already reported (De Rosso et al., 2009) that the highest amounts of polyphenols released from woods into alcoholic solutions miming wine, were from chestnut wood. The same study also referred robinia to be the poorest one, regarding polyphenols release measured by Folin Ciocalteau reagent. Looking into toasting effect, the results show that heavy toast is clearly the main responsible for reducing the amount of phenolic compounds released into synthetic wine solutions.
TABLE II
Total polyphenols and absorbance at 280 nm of hydroalcoholic extracts of the four wood species subjected to different toasting levels
Polifenois totais e absorvância a 280nm dos extratos hidroalcoólicos das quatro espécies sujeitas a diferentes níveis de queima
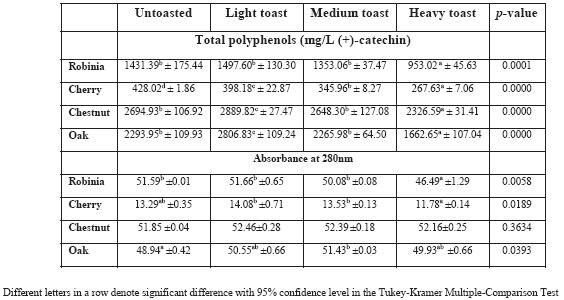
Table II also lists the absorbance of hydroalcoholic extracts measured at a wavelength of 280nm. Values obtained for absorbance at 280nm are in accordance with values of polyphenols determined by oxidation with Folin Ciocalteau reagent. Cherry presents smaller values for absorbance at 280 nm and chestnut presents the highest values. The total phenolic compounds in enology are often measured by these two methods: absorbance at 280nm and/or the Folin Ciocalteau reaction. Both methods are used to provide information regarding the polyphenol content of the matrix but should be aware that both methods are not specific for phenolic compounds. In particular, for the measurement of the absorbance at 280 nm will contribute all the compounds, phenolics or not, that absorb at this wavelength. However, polyphenols which maxima absorbance is not 280 nm will not be measured using this method (Derkyi et al., 2011). In the case of Folin Ciocalteau method, which takes into account all hydroxyl aromatic compounds, commonly used to determine the content of phenolics, namely soluble phenolics (such as anthocyanins) as well as complex phenolics (such as hydrolysable and condensed tannins), the phenolic contents values are over- or underestimation when compared to other methods (Vermerris and Nicholson, 2006). This could explain the differences between phenolic contents obtained using the two methods described above. However, those differences reflect the impact of the wood composition which is infl uenced by botanical species.
Phenolic compounds extracted to the hydroalcoholic solutions by the different types of wood are presented in Tables III and Table IV. When ANOVA was applied to these data significant differences were observed, which are closely related with the toasting level.
TABLE III
Phenolic compounds in hydroalcoholic solution with Robinia and Chestnut chips*
Compostos fenólicos nos extratos hidroalcoólicos com aparas de Robinia e Castanheiro
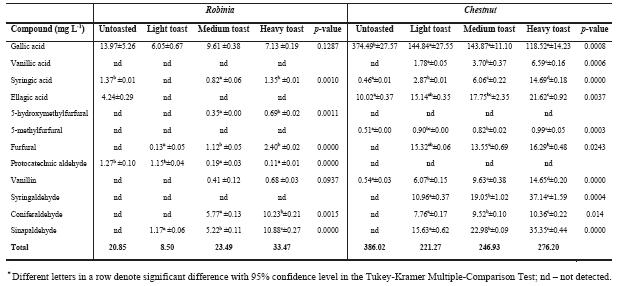
TABLE IV
Phenolic compounds in hydroalcoholic solution with cherry and oak chips*
Compostos fenólicos nos extratos hidroalcoólicos com aparas de cerejeira e carvalho
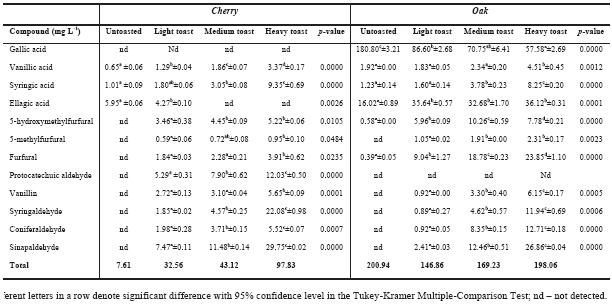
Among the extractable substances, some of the oenological important compounds are the gallotaninns and ellagitannins. Heat treatments (seasoning and toasting) can cause decomposition of these tannins giving rise to gallic and ellagic acid, respectively while vanillic and syringic acids come from lignin degradation (Puech et al., 1989). For hydroalcoholic extracts of chestnut, oak and cherry chips, there is a significant increase of these phenolic acids with the rise of toasting level, except for gallic acid. Gallic acid presents its highest value in untoasted oak and chestnut chips, meaning that with high temperature gallic acid is degraded. Similar result has been described by others authors (Gimenez-Martinez et al., 1996; Canas et al., 2003). According to our results, gallic acid is absent in the hydroalcoholic solutions of cherry chips. In particular for robinia extracts, our results seems to be inconclusive concerning the influence of the toasting level on the phenolic acids contents since this type of wood posseses a lack of these compounds. We can also conclude that ellagic acid presents a more erratic behaviour among samples. Concerning ellagic acid contents, the increment observed in oak and chestnut does not occur with cherry and robinia. It can be linked to different contents of gallotannins and elagitannins, as these woods have released very small amounts of gallic acid. Regarding phenolic compounds, so important in oak wood, the absence of hydrolysable tannins in cherry heartwood was already noticed (Sanz et al., 2010).
The presence of furanic aldehydes in woods is link to sugar thermo-degradation. 5-hydroxymethylfurfural and 5-methylfurfural come mainly from hexoses, existing in cellulose, and furfural comes from pentoses, main constituents of hemicelluloses (Hodge, 1967).
Several authors refer that untoasted oak wood present small amounts or furfural, 5-methylfurfural (Nabeta et al., 1986; Marsal and Sarre, 1987; Chatonnet et al., 1989; Marco et al., 1994; Gétaz et al., 1996; Garcia-Romero et al., 1998; Pérez-Coello et al., 1999) and 5-hydroxymethylfurfural (Artajona, 1991; Masson et al., 2000). Seasoning seems to play a role in hexoses and pentoses degradation, but toasting effect is stronger.
Previous studies already suggested (Chatonnet et al., 1989; Masson et al., 2000; Bourgois and Guyonnet, 1988) that furfural is dominant in toasted oak wood due to the fact that hemicelluloses are highly thermo unstable. Controversy seems to be related to the impact of toasting levels in the final amounts of furfural, some (Chatonnet et al., 1989) reported reaching the highest levels at medium toast while others clearly relate the increasing levels related to the increase in toasting levels (Nomdedeu et al., 1988; Artajona, 1991; Canas et al., 2003). These different statements are, most likely, because toasting temperatures is not unanimously related to a toasting level definition.
In our case, except with cherry chips, furfural was, as well, the furanic derivative present in higher amounts after toasting.
Untoasted wood types release low contents of phenolic aldehydes (vanillin, syringaldehyde, coniferaldehyde and sinapaldehyde) into alcoholic solutions; toasting originates the increment of these compounds (Chatonnet et al., 1989; Artajona, 1991; Nomdedeu et al., 1988; Nishimura et al., 1983; Dubois, 1989; Sarni et al., 1990a; Mosedale and Ford, 1996; Canas et al., 2003). In our alcoholic solutions, regardless the wood species, we can observe a signifi cant increase in these compounds with toasting level. These compounds come from lignin degradation (Puech et al., 1989; Puech et al., 1990) during thermal processing of woods. Heat treatment during the toasting process leads to a decarboxilation and cleavage of the aryl-alkyl-ether bondages of terminal units of lignin, with formation of cinnamic aldehydes, like coniferaldehyde and sinapaldehyde. When using higher temperature, to obtain heavy toast chips, oxidative cleavage on C-C skeleton of these aldehydes may occur, leading to the corresponding benzoic aldehydes, vanillin and syringaldehyde (Sarni et al., 1990b; Chatonnet, 1995). Phenolic aldehydes can also be thermo degraded into phenolic acids or volatile phenols (Chatonnet, 1995). Our results show a continuous increase in phenolic aldehydes released from woods into alcoholic solution, regardless wood species; it seems that temperature applied, in heavy toast, was not enough to promote cinnamic aldehydes degradation.
Syringaldehyde and sinapaldehyde in hydroalcoholic solutions, with toasted woods, are more abundant than vanillin and coniferaldeyde, except for robinia. This seems to be related to the structure of these phenolic aldehydes. The former have a syringylpropane structure that has a higher thermal stability than the latter, which presents a guaiacylpropane structure (Canas et al., 2011).
Protocatechuic aldehyde was only found in hydroalcoholic solution from robinia and cherry woods presenting a different behaviour. In the case of robinia, a slight decrease with the increase of toasting level was observed while for cherry a dramatic increase occurred. The formation of protocatechuic aldehyde, during toasting, is explained by heat degradation of quercetin producing the correspondent acid as a cleavage reaction product (Buchner et al., 2006); further heat reduction will result in aldehyde formation (Sanz et al., 2010). In spite the presence of both acid and aldehyde protocatechuic in cherry heartwood seasoned and toasted that were found by Sanz et al., (2010) in our work no protocatechuic acid was found.
Comparing these four wood species, we might conclude that some main compounds are common to all of them, namely gallic and ellagic acids and the aldehydes vanillin, syringaldehyde, coniferaldehyde, sinapaldehyde, as well as the furanic derivatives furfural, 5-hydroxymethylfurfural and 5-methylfurfural. Furanic derivatives were only identified by means of their UV–Vis spectra and retention times. Except for these furanic derivatives, the respective [M-H]- deprotonated molecule was the base peak in the MS pattern. Gallic acid also gave [M-H-44]-fragment ion via loss of a CO2 group from the carboxylic acid moiety (table 1). For the methoxylated aldehydes (vanillin, syringaldehyde and coniferaldehyde) the mass spectrum shows the respective deprotonated molecules at m/z 151, 181 and 177, respectively. The mass spectrum of the more retained hydroxycinnamic aldehyde (sinapaldehyde) gave also a deprotonated molecule [M-H]-at m/z 207, although the main fragment present is due to the loss of a methyl group. Fragmentations were confirmed with the authentic standards.
Considering the above findings, it seems that attention should be drawn to the kinetics of release and trace compounds identifications. Oak and chestnut release higher amounts of low molecular weight phenolic compounds into model solutions. These two wood species show an absorbance spectra and polyphenols content very similar. Cherry and robinia have different behaviour. Cherry released the lowest amount of compounds into the model solution presenting, after extraction, the smaller amounts of phenolic compounds and the lower absorption at 250-450 nm. Data found for robinia wood is rather different: very small amounts of phenolic compounds were identified but absorbance at 280nm may suggest the presence of other phenolic compounds in the extracts (Fig. 1). In fact, Figure 2 depicted a selected region of LC-DAD chromatogram of robinia extracts, showing a peak at 22,38 minutes in untoasted sample (A), that could be assigned to dihydrorobinetin, according to spectroscopic (λ max (nm): 276, 312sh) and spectrometric data: m/z (%) [attribution] 303 (80) [M- H]-, 285 (20) [M-HH2O]-. Same selected region for heavy toast sample (B) shows the appearance of another peak at 23,24 minutes. Based on spectroscopic (λ max (nm): 282, 325sh) and spectrometric data: m/z (%) [attribution] 303 (100) [M- H]-, 285 (20) [M-H-H2O]-, this compound, having a similar structure may be assigned to a dihydroflavonol analog. Roux and Paulus (1962) first reported Dihydrorobinetin in non toasted robinia heartwood using paper chromatography. Recently, Cerezo et al. (2009) also reported this compound in wine vinegar aged in acacia wood and Sanz et al. (2011) reported it in acacia heartwood. According to these authors and our own results, it seems to be more characteristic of untoasted robinia wood.
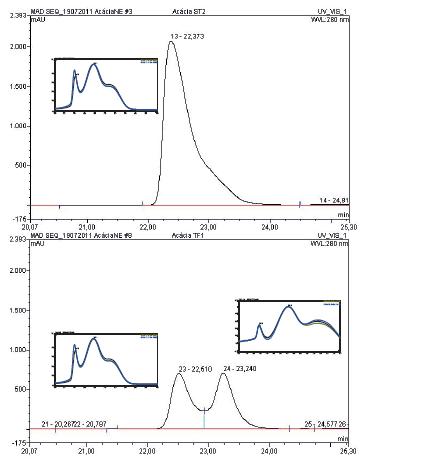
Figure 2 – LC-DAD chromatogram at 280 nm from a robinia extract: A untosted chips, B heavy toasted chips
Cromatograma LC- DAD a 280 nm de extratos de robinis: A aparas não queimadas, B aparas com nível de queima forte
CONCLUSIONS
Chip size and wood structure affect the release of compounds from wood into synthetic wine. As we used similar chip sizes it seems that wood structure rather then size affect extraction efficiency. Eachwood species has a different profile regarding phenolic acids, phenolic aldehydes and furanic derivatives, but it is also clear that the effect of toasting is similar. Increasing toasting levels lead to an increase of phenolic aldehydes and phenolic acids such as 996.13syringic and vanillic acids. More studies are need regarding robinia phenolic composition, as it seems to have other phenolic compounds, which explain the high values obtained for absorbance measures and total polyphenolic content. The optimization of the amount of chips used may, eventually, allow achievingresults similar to the oak chip application.
REFERENCES
Artajona J., 1991. Caracterisation del roble según su origen y grado de tostado, mediante la utilizacion de GC y HPLC. Viticultura/ Enologia Profesional, 14, 61-72. [ Links ]
Bourgois J., Guyonnet R., 1988. Caracterization and analysis of torrefi ed wood. Wood Science and Technology, 22, 143-155. [ Links ]
Buchner N., Krumbein A., Rohn S., Kroh L.H., 2006. Effect of hermal processing on the flavonols rutin and quercitin. Rapid. Communications in Mass Spectrometry, 20, 3229-3235. [ Links ]
Canas S., Belchior A.P., Spranger M. I., Bruno-de-Sousa R., 2003. High performance liquid chromatography method for analysis of phenolic acids, phenolic aldehydes and furanic derivatives in brandies. Development and validation. Journal of Separation Science, 26, 496-502. [ Links ]
Canas S., Belchior A.P., Spranger M.I., Bruno-de-Sousa R., 2011. HPLC method for the quantification of phenolic acids, phenolic aldehydes, coumarins and furanic derivatives in different kinds of toasted wood used for the ageing of brandies. Analytical Methods, 3, 186-191. [ Links ]
Cerezo A.B., Espartero J.L., Winterhalter P., Garcia-Parrilla M.C., Troncoso A.M., 2009. (+)-Dihydrorobinetin: a marker of vinegar aging in acacia (Robinia pseudoacacia) wood. Journal of Agricultural and Food Chemistry 57, 9551-9554. [ Links ]
Chatonnet P., Boidron J.N., Pons M., 1989. Incidence du traitement thermique du bois de chêne sur sa composition chimique. 2ºpartie: Évolution de certains composés en fonction de l´intensité de brûlage. Connaissance de la Vigne et du Vin, 23, 223-250. [ Links ]
Chatonnet P., 1995. Influence des procédés de tonnellerie et des conditions d´élevage sur la composition et la qualité des vins élévés en fût de chêne. Pp. 268. Thèse Doctorat. UFR Institut d´Oenologie, Université de Bordeaux II, Bordéus. [ Links ]
Citron G., 2005. Uso del legno in enologia: specie botaniche utilizzate, anatomia e classificazione. L’Informatore Agrario, 59, 69-72.
Derkyi N.S.A., Adu-Amankwai B., Sekyere D., Darkwa N.A., 2011. Rapid prediction of extractives and polyphenolic contents in Pinus caribara bark using near infrared refl ectance spectroscopy. International Journal of Applied Sciences, 1, 1- 11. [ Links ]
De Rosso M., Cancian D., Panighel A., Vedova A.D., Flamini R., 2009. Chemical compounds released from five different woods used to make barrels for aging wines and spirits: volatile compounds and polyphenols. Wood Science and Technology, 43, 375-385. [ Links ]
Dubois P., 1989. Apport du fût de chêne neuf à l’arôme des vins. Revue Française d’Oenologie, 120, 19-24.
Fernández de Simón B., Cadahía E., Del Álamo M., Nevares I., 2009. Volatile compounds in acacia, chestnut, cherry ash and oak woods, with a view to their use in cooperage. Journal of Agricultural and Food Chemistry, 57, 3217-3227. [ Links ]
Fernández de Simón B., Cadahía E., Muiño I., Del Álamo M., Nevares I., 2010. Volatile composition of toasted oak chips and staves and of red wine aged with them. American Journal of Enology and Viticulture , 61, 157-165. [ Links ]
Garcia-Romero E., Pérez-Coello M.S., Sanz J., Cabezudo M.S., 1998. Quantitative analysis of the principal volatile compounds in oak wood by direct thermal desorption (DTD) and GC/MS. Analusis, 26, 33-35. [ Links ]
Gétaz J., Canetti J., Lebedeff J., Horisberger D., 1996. Utilisation de chênes indigènes pour la construction de barriques: une solution d´avenir. Revue suisse de viticulture, d’arboriculture et d’horticulture, 28, 183-194.
Gimenez-Martinez R., De La Serrana H.L.G., Mir M.V., Granados J.Q., Martinez M.C.L., 1996. Influence of wood heat treatment, temperature and maceration time on vanillin, syringaldehyde, and gallic acid contents in oak wood and wine spirit mixtures. American Journal of Enology and Viticulture, 47, 441-446. [ Links ]
Hodge J.E., 1967. Non-enzymatic browning reaction. In W. Schultz, E. A. Day & L. M. Libbey (Eds.), Symposium on Foods. Chemistry and Physiology of flavors. (pp. 465-491). Westport, USA: AVI Publishing. [ Links ]
Madrera R.R., Valles B.S., García Y.D. Argüelles P., Lobo A.P., 2010. Alternative woods for aging distillates-an insight into their phenolic profiles and antioxidant activities. Food Science and Biotechnology, 19, 1129-1134. [ Links ]
Marco J., Artajona J., Larrechi M.S., Rius F.X., 1994. Relationship between geographical origin and chemical composition of wood for oak barrels. American Journal of Enology and Viticulture, 45, 192-200. [ Links ]
Marsal F., Sarre C., 1987. Étude par chromatographie en phase gaseuse de substances volatiles issues du bois de chêne. Connaissance de la Vigne et du Vin, 21, 71-80. [ Links ]
Masson E., Baumes R., Moutounet M., Puech J.L., 2000. The effect of kiln-drying on the levels of ellagitannins and volatile compounds of European oak (Quercus petraea Liebl.) stave wood. American Journal of Enology and Viticulture, 51, 201-214. [ Links ]
Mosedale J.R., Ford A., 1996. Variation of the flavour and extractives of european oak wood from two french forests. Journal of the science of food and agriculture, 70, 273-287. [ Links ]
Nabeta K., Yonekubo J., Miyake M., 1986. Analysis of volatile constituents of european and japanese oaks. Mokuzai Gakkaishi, 32, 921-927. [ Links ]
Natali N., Chinnici F., Riponi C., 2006. Characterization of vola-tiles in extracts from oak chips obtained by accelerated solvent extraction (ASE). Journal of Agricultural Food Chemistry, 54, 8190-8198 [ Links ]
Nishimura K., Ohnishi M., Masuda M., Koga K., Matsuyama R., 1983. Reactions of wood components during maturation. In J. R. Piggott (Eds) Flavour of distilled beverages: origin and development (pp. 241-255). Chichester, UK: Ellis Horwood. [ Links ]
Nomdedeu L., Leaute R., GrandChamp B., Bonnichon C., Laurichesse D., Trichet P., 1988. Brûlage des barriques de chêne et qualité des vins de Médoc. Progrès Agricole et Viticole, 105, 505-556. [ Links ]
Organisation Internationale de la Vigne et du Vin. 2005. Morceaux de bois de chêne. Résolution N°3 du 20 juillet 2005, OENO 3/2005. [ Links ]
Pérez-Coello M.S., Sanz J., Cabezudo M.D., 1999. Determination of volatile compounds in hydroalcoholic extracts of french and american oak wood. American Journal of Enology and Viticulture, 50, 162-165. [ Links ]
Puech J.L., Robert A., Mouttet B., 1989. Evolution of oak wood lignin subjected to fl ash hydrolysis. Holzforschung, 43, 235-238.
Puech J.L., Sarni F., Labidi A., Mouttet B., Robert A., 1990. Delignification of oak wood with an ethanol-water solution in a fl ow-through reactor. Holzforschung, 44, 367-371.
Roux D.G., Paulus E., 1962. Condensed tannins: 13. Interrelationships of flanonoid components from the heartwood of Robinia pseudacacia. Biochemistry Journal, 82, 324-330. [ Links ]
Sarni F., Rabier P., Moutounet M., 1990a. Fabrication des barriques et thermotraitements: relevé des températures. Revue Française d’Oenologie, 123, 53-58.
Sarni F., Moutounet M., Puech J.L., Rabier P., 1990b. Effect of heat treatment of oak wood extractable compounds. Holzforschnung, 44, 461-466. [ Links ]
Sanz M., Cadahía E., Esteruelas E., Munoz A.M., de Simon B.F., Hernández T., Estrella, I.J., 2010. Phenolic Compounds in Chestnut (Castanea sativa Mill.) Heartwood. Effect of Toasting at Cooperage. Journal of Agricultural and Food Chemistry, 58, 9631- 9640. [ Links ]
Sanz M., de Simon B.F., Esteruelas E., Munoz A.M., Cadahía E., Hernández T., Estrella I.J., Pinto E., 2011. Effect of toasting intensity at cooperage on phenolic compounds in acacia (Robinia pseudoacacia) heartwood. Journal of Agricultural and Food Chemistry, 59, 3135-3145. [ Links ]
Singleton V.L., Rossi J.A., 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144-158. [ Links ]
Van Jaarsveld F.P., Hattingh S., Minnaar P., 2009. Rapid induction of ageing character in brandy products – Part III. Influence of toasting. South African Journal of Enology and Viticulture, 30, 24-37.
Vermerris W., Nicholson R., 2006. Isolation and identifi cation of phenolic compounds - A practical guide. In W. Vermerris & R. Nicholson (Eds.), Phenolic Compound Biochemistry (pp. 151-196). Dordrecht, Netherlands: Springer. [ Links ]
ACKNOWLEDGEMENTS
This work is funded by FEDER Funds through the Operational Programme for Competitiveness Factors - COMPETE and National Funds through FCT - Foun-dation for Science and Technology under the StrategicProject PEst-C/AGR/UI0115/2011. Authors wish to thanks the Cooperage J.M. Gonçalves Tanoaria, Lda (Palaçoulo, Portugal) for supplying the wood chips
* Corresponding author: T. 351 266 769869; F. 351 266 769828, e-mail: mjbc@uevora.pt
(Manuscrito recebido em 24.04.2012. Aceite para publicação em 12.06.2012)













