Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
Compartilhar
Silva Lusitana
versão impressa ISSN 0870-6352
Silva Lus. v.18 n.2 Lisboa 2010
Relationships of Selected Soil Parameters and Natural Pastures Yield in the Montado Ecosystem of the Mediterranean Area Using Multivariate Analysis
Eugénio Mendes Ferreira*, Nuno Simões**, Isabel Videira e Castro* and Luís Carneiro*
*Senior Researchers
Instituto Nacional de Investigação Agrária/INRB, IP. Av. da República, Quinta do Marquês, 2780-159 OEIRAS
**Researcher
Instituto Nacional de Investigação Agrária/INRB, IP. Apartado 6, 7350-951 ELVAS
Abstract
Chemical, physical and biological soil parameters (OM, total N, pH, P2O5, K2O, Mg, B, WHC, free–living nitrogen fixing and rhizobial microflora properties) and pastures yield were characterized and used to determine relationships in 40 locations covering different growth conditions of the "montado" ecosystem. Soil samples were collected in Spring. Reasonable soil fertility was found. The soils presented, in general, intermediate values for OM, K2O, Mg, and Bo and low values for pH and P2O5. Populations of free-living nitrogen fixing bacteria were high, with an average of 4.2 x 107 CFU g-1 of soil, being nitrogenase activity highly variable, with an average of 34 nmoles of C2H4 g-1 of soil h-1. Rhizobial population associated to Trifolium subterraneum was high, with an average of 106 bacteria g-1 of soil, having adequate nitrogen fixing potential for the majority of the soils. Rhizobial population associated to Medicago polymorpha was low, with an average of 6.5 x 104 bacteria g-1 of soil, an insufficient value for a good nodulation, having low nitrogen fixing potential for the majority of the soils. Annual yield of natural pastures varied highly among locals, with an average of 3245 kg ha-1, a usual value for this ecosystem. In general, soil properties were independent of the soil samples. The chemical parameters OM, P2O5, K2O and total N were important factors to pasture yield. A great variability, even in soil samples collected within a short distance, was observed. A relationship between geological origin and productivity was found, being the granitic soils more productive in non-legume plants.
Key words: Montado ecosystem; pasture yield; soil parameters; sustainable agriculture
Relações Entre Parâmetros Seleccionados de Solo e Produção de Pastagens Naturais no Ecossistema Montado da Região do Mediterrâneo Através de Análise Multivariada
Sumário
Parâmetros químicos, físicos e biológicos do solo (MO, N total, pH, P2O5, K2O, Mg, B, CR, e as propriedades da microflora fixadora de azoto de vida livre e dos rizóbios) e a produção de pastagens foram caracterizados e utilizados para determinar as relações em 40 locais abrangendo diferentes condições de crescimento dentro do ecossistema "montado". As amostras de solo foram colhidas na Primavera. Uma razoável fertilidade dos solos foi encontrada; apresentando em geral valores intermédios para a MO, K2O, Mg e Bo e valores baixos para o pH e P2O5. As populações de bactérias fixadoras de azoto, de vida livre, foram elevadas, com uma média de 4.2 x 107 UFC g-1 de solo, com uma actividade da nitrogenase altamente variável e com uma média de 34 nmoles de C2H4 g-1 de solo h-1. A população rizobiana associada ao Trifolium subterraneum foi elevada, com uma média de 106 bactérias por grama de solo, tendo um potencial de fixação de azoto adequado para a maioria dos solos. A população rizobiana associada à Medicago polymorpha foi baixa, com uma média de 6.5 x 104 bactérias por grama de solo, um valor insuficiente para uma boa nodulação (no Outono), com um baixo potencial fixador de azoto para a maioria dos solos. A produtividade anual das pastagens naturais variou muito entre os locais, com uma média de 3245 kg ha-1, um valor normal para este ecossistema. Em geral, as propriedades do solo foram independentes das amostras de solo. Os parâmetros químicos MO, P2O5, K2O e N total foram factores importantes para a produção das pastagens. Uma grande variabilidade nas amostras de solo colhidas a uma curta distância foi observada. Uma relação entre a origem geológica e a produtividade foi também verificada, sendo os solos graníticos mais produtivos para as plantas não-leguminosas.
Palavras-chave: Ecossistema montado; produção de pastagem; parâmetros do solo; agricultura sustentável
Relations de Paramètres Sélectionnés des Sols et la Production des Pâturages Naturels Chez l'Écosystème «Montado» de la Région Méditerranéenne en Utilisant une Analyse Multivariée
Résumé
Paramètreschimiques, physiques et biologiques des sols (MO, N total, pH, P2O5, K2O, Mg, B et propriétés de la microflore des microorganismes fixateurs de azote, vivant en vie libre, des Rhizobium) et la production des pâturages ont été caractérisés et utilisés pour déterminer les relations des 40 situations couvrant différentes conditions de croissance parmi l'écosystème "montado". Les terres ont été récoltées et analysées au Printemps. La fertilité des sols a été raisonnable, présentant en général, des valeurs intermédiaires pour la MO, K2O, Mg, et Bo et des valeurs faibles pour pH et P2O5. Les populations de bactéries fixatrices de azote, non symbiotiques, ont été élevées, avec une moyenne de 4.2 x 107 de colonies g-1 de sol, la nitrogénase était très variable, avec une moyenne de 34 nmoles de C2H4 g-1 de sol h-1. La population des rhizobia associés au Trifolium subterraneum était élevée, avec une moyenne de 106 bactéries g-1 de sol, ayant un potentiel adéquat de fixation d'azote pour la majorité des sols. La population des rhizobia associés à Medicago polymorpha était faible avec une moyenne de
6.5 x 104 bactéries g-1 de sol, une valeur insuffisante (à l'Automne) pour une bonne nodulation, ayant un faible potentiel de fixation pour la majorité des sols. La production annuelle des pâturages naturels a varié beaucoup selon les situations, avec une moyenne de 3245 kg ha-1, une valeur normale pour cet écosystème. En général, les propriétés du sol sont indépendantes des situations. Les paramètres chimiques MO, P2O5, K2O et N total ont été des facteurs importants pour la production des pâturages. Une grande variabilité, même dans les situations de sols prélevés à une courte distance, a été observée. Une relation entre l'origine géologique et la productivité a été trouvée, les sols granitiques étant plus productifs pour les plantes non-légumineuses.
Mots clés: Écosystème montado; production des pâturages; paramètres du sol; agriculture soutenable
Introduction
The "montado" is an agroforestry and pasture system of the regions of Southern of Iberian Peninsula, Portugal and Spain where it is called "dehesa", associated to extensive and sustainable livestock production under scattered oak trees, Quercus suber and Quercus ilex. It is the most extended agroforestry system in Europe, with more than 3 million hectares. This system has been known through centuries for its multiple use and renewable resources, mainly silvo-pastoral and cereal cropping (JOFFRE et al., 1988). When well established it can assume different functions for the ecosystem, such as optimisation of available energy through biomass production, soil preservation, nutrients circulation, water conservation and bioregulation of climate or microclimate stability (Trujillo and Mata, 2001).
In Portugal, this special ecosystem supports a sustainable extensive agriculture, where the natural pastures and the introduced legume based pastures play an important role, supporting the direct grazing by cows and sheep. Natural pastures are in their majority poor and managed with a low animal-stocking rate due the low soil fertility, associated to a diverse, but few productive natural flora.
Soil chemical and physical properties are usually associated to soil productivity providing information that can be used by land managers to make management decisions. In this rain-fed ecosystem, the nitrogen fixation by free-living bacteria and legume symbioses is a major process of providing nitrogen to the soils, being legumes an important component of a strategy for increasing productivity and sustainability of farming systems. The amount and effectiveness of background rhizobial populations are fundamental parameters for evaluation the need for legume seeds inoculation, procedure that can be determinant for improving pastures yield and quality.
Our objectives were to assess some soil properties, to determine relationships among chemical, physical and biological soil parameters, sampling local, soil origin and natural pastures yield under Quercus suber and Quercus ilex trees on the Southern of Portugal. These results will allow the identification of parameters that can be used for development of practices for increasing soil quality and crop production.
Materials and methods
Study site
The study was carried out on a range of long term dryland natural pastures, at the "montado" ecosystem (Alentejo-Portugal). This ecosystem covers an area superior to 106 ha. The climate is characterized by dry hot summers and wet cool winters with an average annual temperature ranging from 14 to 17ºC. Rainfall is very variable, with an average annual precipitation ranging from 500 to 700 mm (450-600 during the agricultural year of experiment, October-September), falling the majority during the cool season (October-April). Soils, mainly derived from granite and schist, are mostly poor. Lightly hilly lands are the dominant landscape, with average altitudes ranging from 200 to 250 m.
Field plants sampling and pasture yield
In Autumn (October), 13 farms (locals) in different edaphic and climatic environmental conditions were selected for this study. At each farm, outside canopy shade, three exclusion cages (1 x 1m) were placed at upper, mid and lower slope positions, trying to embrace different plant growth conditions. On one farm, due their larger area and different soils, four cages were placed. Dung patches visually affected none of the cages places.
Two cuttings per season were undertaken, one in the end of February (winter) and the other in the end of May (Spring). The shoot material was handcut with shears to a height of 2 cm. The plants were separated into legumes (leg) and non-legumes (n-leg), dried at 70ºC and weight for total yield (TY).
Soil sampling and processing
In May, forty soil samples were collected on 13 farms (40 exclusion cages) covering different growth conditions. Sampling was carried out in spring, when soil biological activity in Mediterranean environmental conditions is higher (GARCIA et al., 1997). Five soil cores from the upper 10 cm of soil, were collected aseptically from each exclusion cage, bulked, and stored in sealed plastic bags. Samples were transported to the laboratory under cooled conditions where they were thoroughly mixed, passed through a 2 mm sieve to remove stones and large pieces of organic matter and stored in the refrigerator (6ºC) before physical, chemical and microbial analysis.
Chemical and physical properties
Chemical and physical analysis of the soil samples: organic matter (OM), total N, pH (H2O), extractable P (P2O5), K (K2O), Mg and B, and water holding capacity (WHC) were done according to the usual procedures of the Analytical Service.
Biological properties
Free-living nitrogen fixing microflora properties
Cultivable nitrogen-fixing bacteria were enumerated by plate counting from colony forming units (CFU) growing on a N-free medium (POCHON and TCHAN, 1948; PARKINSON et al., 1971), containing actidione at 20 mg l-1. The results (Free-liv) were expressed as log of number of colony forming unities (CFU) per gram of dried soil.
Potential nitrogen fixation by free-living microflora was evaluated indirectly by acetylene reduction assay (HARDY et al., 1973), a sensitive method for assaying nitrogenase activity, using the gas chromatography technique. Two seedlings of Lolium multiflorum were grown in glass bottles containing 15 g of soil adjusted to 75% WHC in a controlled environmental growth room (18-20ºC, 12 h day) during 6 weeks. For measurement of acetylene reduction activity (ARA), acetylene was injected at 10% of the bottle volume, after removal of the same volume of air, being the soils exposed to C2H2 for 1 h at 20ºC. The results (ARA Free-liv) were expressed as nanomoles (nmol) of C2H4 per gram of soil and per hour.
Rhizobial microflora properties
Rhizobial microflora associated to Trifolium subterraneum (Rhizobium leguminosarum bv. trifolii) and Medicago polymorpha (Sinorhizobium meliloti) were estimated by the most probable number (MPN) method (VINCENT, 1970) using a ten-fold dilution series with the test plants Trifolium subterraneum cv. Clare and Medicago polymorpha cv. 66, growing in Jensen's agar medium (VINCENT, 1970). The results were expressed as log of number of rhizobia bacteria (Rh trifolii for clover and Rh meliloti for annual medic) per gram of dried soil (Table 1). The plants were grown for 8 weeks in a controlled environmental growth room (18-20ºC, 12 h day) and harvested. The roots were examined for nodulation and the dry matter from the shoots was measured after oven drying at 70ºC. The "whole-soil inoculation technique" (BROCKWELL et al., 1988; QUIGLEY et al., 1997) was used to assess the nitrogen fixing potential of the soil rhizobial populations. The results obtained from dry matter production of the nodulated plants (Trifolium subterraneum - DM Trifolium and Medicago polymorpha - DM Medicago), from the soil dilutions 10-1 and 10-2, allowed to differentiate the rhizobial resident populations.
Statistical analyses
The data were analysed using multivariate analysis: Principal component analysis (PCA), using NTSYS-pc version 2.1 (ROHLF, 2000) and discriminant analysis (DA) using NCSS software package (HINTZE, 2001).
Analyses were conducted on the complete dataset for the 40 soil samples characterized by 16 properties (variables) (Table 1). The principal component analysis allows us to classify the soil samples and to obtain different similarity groups and to find relationships among variables. The discriminant analysis allows us to validate the existence of geographical or geological patterns of soil samples distribution.
Table 1 - Soil chemical, physical and biological parameters and pasture yield (legumes and non-legumes)
Results and discussion
Pasture yield
Annual yield of natural pastures varied highly among locals (Table 1). The shoot material ranged from 0 to 1257 kg ha-1 with an average of 404 kg ha-1 for legumes (TY leg) and from 255 to 6255 kg ha-1 with an average of 2842 kg ha-1 for non-legumes (TY n-leg). Total yield ranged from 287 to 6866 kg ha-1 with an average of 3245 kg ha-1.
Several authors obtained values for unfertilised annual pastures yield in this ecosystem: In Portugal, the yield of natural pastures was evaluated in different conditions; PARREIRA and GARRIDO, 1987, found values of 603, 951 and 1584 kg ha-1 (average of 3 years) for pastures yield on 3 different soils. LOURENÇO et al., 1994, on 4 different soils, obtained values of 512, 532, 780 and 1201 kg ha-1 for the yield (average of 3 years) under the oak trees, and values of 596, 627, 672 and 1418 kg ha-1 in the open area. In the Spanish "dehesa", MORENO et al., 2007, obtained values ranging between 790 and 1774 kg ha-1. MORENO, 2007, obtained values ranging between 670 and 1033 kg ha-1. OLEA and SAN MIGUEL-AYANS, 2006, referred general values for pastures productivity, ranging from 1000 to 2700 kg ha-1.
In this study we found pasture yields that confirm those results, being our results slightly superior, possibly as a consequence of a regular annual rainfall (450-600 mm).
Chemical and physical properties
Values for the soil variables presented in Table 1 showed a wide range either among the sampling locals or the samples from the same local. According to the recommended levels for crops fertilisation in Portugal (Laboratório Químico Agrícola Rebelo da Silva 2000) the soils presented in general, intermediate values for OM, K2O, Mg, and B and low values for pH and P2O5.
In spite of the acceptable soil fertility in the "montado" ecosystem, it can be easily increased using a phosphorus and calcium based fertilizer, as for example the calcium superphosphate (18% P2O5).
Biological properties
Free-living nitrogen fixing microflora properties
Free-living nitrogen-fixing microbial populations in the natural ecosystem are crucial for the ecosystem maintenance and productivity due to the special role of these organisms in the global biogeochemical cycle of nitrogen (KAVADIA et al., 2007). It is known that free-living nitrogen-fixing populations in soil are affected by environmental, physical and chemical conditions, including the organic components excreted by plant roots, as well as by interactions with other microbial populations.
Values of cultivable free-living nitrogen fixing bacteria, presented in Table 1, were high for all soils, ranging from 6.29 x 106 to 2.95 x 108 CFU g-1 of soil, with an average of 4.2 x 107. These high values were probably due to the time of soil collection (spring), to enough carbon sources (acceptable values for OM) and to the presence of roots and root exudates, which supply the microorganisms with the energy required for their growth and activity. Other authors working in different ecological conditions also obtained results in a wide magnitude. In a study with soil from an arable field in Portugal, OLIVEIRA and PAMPULHA, 2006, found numbers ranging from 3.5 x 104 to 9.5 x 104 CFU g-1 of soil. In Senegal, CHOTE et al. (2002), using soils from 3 and 19 years old natural fallows under Pennisetum found numbers of 1.8 x 106 and 4 x 106 CFU g-1 of soil, respectively. In Brasil, SOARES et al. (2006), studying the nitrogen fixing bacterial community associated with oat (Avena sativa) in different soil management systems of Rio Grande do Sul State, found values ranging from 1.6 x 10 6 to 4 x 1011 CFU with an average of 2 x 106. XIE and STEINBERGER, 2002, in the Negev Desert (Israel) found numbers between 3.9 x 106 and 2.85x107 CFU g-1 of soil in a loessial soil and between 0.7 x 106 and 1.07 x 107 CFU in a sandy soil. Also in a desert in Mexico, RODRÍGUEZ-ZARAGOZA et al., 2008, in soils under perennial shrubs, found numbers ranged from 1.1 x 107 CFU in the surface layer (0-10 cm) to 1.9 x 104 CFU in a deeper layer (40-50 cm).
ARA was highly variable among the soil samples (Table 1), ranging from 0 to 170 nmoles of C2H4 g-1 of soil h-1, with an average of 34 nmoles of C2H4 g-1 of soil h-1, being detected higher rates of ARA in schistose soils.
CHOTE et al., 2002, working on 3 and 19 years old natural fallows, found a potential N2 fixation of 6.4 and 110 nmoles of C2H4 g-1 of soil day-1, respectively.
RODRÍGUEZ-ZARAGOZA et al., 2008, in soils under perennial shrubs in a desert in Mexico, found rates of ARA between 20.1 and 29 nmoles of C2H4 g-1 of soil day-1.
Working with soil cores (6 cm diam x 15 cm length) containing grasses, obtainned from 25 sites in Texas, USA, MORRIS et al., 1985, found ARA rates ranging from 0 to 7.6 mmol of C2H4core-1 day-1. Also in Texas, WRIGHT and WEAVER, 1981, had found ARA rates ranging from 0 to 5.5 mmol of C2H4 core-1 day-1, in root-soil cores (8 cm diam x 15 cm length) collected from forage grasses.
Nitrogenase activity associated with several grass species was measured by THOMPSON et al., 1984, in soil cores (12.5 cm diam x 15 cm length) at 67 sites in New South Wales (Australia) finding a maximum of 246 nmol of C2H4 core-1day-1. Also in Australia, in soil-plant cores (12 cm diam x 16.5 cm length), WEIER, 1980, obtained media values for ARA ranging from 5.3 to 17.2 mmol of C2H4 core-1 day-1, for three grass species in pasture soil.
Correlations between populations of free-living nitrogen fixing microflora (in field conditions) and ARA (in laboratory controlled conditions) are poor and clear explanations cannot be given for the relatively low rates of ARA, but high amounts of CFU. This discrepancy can be a result of various factors including the plate count method used (it determines only the cultivable bacteria), the different plant rhizospheres and the environmental soil conditions used for counting the populations of free–living nitrogen fixing microflora and for ARA evaluation. However, in general terms, larger populations of free-living nitrogen fixers suggest considerable nitrogen fixation (XIE and STEINBERGER, 2002). Microflora around the roots is largely dependent of organic nutrients from the roots exudates and the factors controlling exudation also control nitrogenase (DOMMERGUES et al., (1973). Different plant species induce different environment (favourable or adverse) for nitrogenase activity in the rhizospheres. Also the plant growth stages and the physical, biological and chemical soil characteristics can influence the nitrogenase activity.
Rhizobial microflora properties
Subterranean clovers and annual medics play a major role in supplying nitrogen to dryland pastures on the Southern of Portugal, place where these legume plants occur naturally. The numbers of the nodulating indigenous rhizobial populations and their symbiotic effectiveness with the hosts are fundamental in satisfying the nitrogen requirements of the legume plants, but a considerable variation of the number of rhizobia in soils and a ample range of symbiotic effectiveness can be found. Agricultural soils are often constrained in their ability to sustain productive farming systems due to factors associated to low fertility and water stress, that can have a negative impact on the legume-Rhizobiumsymbiotic relationship.
The size of rhizobial populations fluctuates through the year with higher numbers in spring and lower numbers in autumn, after the dry summer (sowing time). These fluctuations can be marked, 10-100 times more at spring than at autumn (SLATTERY and COVENTRY, 1993). When analysing our results, this seasonal variation, verified in similar environment, must be taken in account and a reduction of 50 times for rhizobial populations at sowing time can be realistic.
Results from this study, presented in Table 1, showed that, in spring, the indigenous rhizobial populations associated to Trifolium subterraneum, were in general high, ranging from 0 (only 1 soil sample) to 1.15 x 107 rhizobia bacteria per gram of soil, with an average of 106. In a general way, the size of the rhizobial populations (5 x 104 in autumn) are enough for a good nodulation of the subterranean clovers and the introduction of new and more efficient rhizobial strains can be difficult and often unsuccessful (BROCKWELL, 1981; HERRIDGE et al., 2002; SLATTERY and PEARCE, 2002). The nitrogen fixing potential of the rhizobial populations associated to clover was adequate for the majority of the soils, originating a dry mater production similar to the inoculated control. In these conditions, the introduction of new clover seeds implies their inoculation with a large number of bacteria, superior to 105 per seed, using competitive and efficient rhizobial strains.
The rhizobial populations associated to Medicago polymorpha (annual medic), even in spring, were low, ranging from 4 x 10 to 106 (only 1 soil sample) rhizobia bacteria per gram of soil, with an average of 6.5 x 104, (1.3 x 103, in autumn) being in their majority inferior to 103, an insufficient value for a good nodulation (BROCKWELL, 1981; HERRIDGE et al., 2002; SLATTERY and PEARCE, 2002). The nitrogen fixing potential of the rhizobial populations associated to annual medics was low for the majority of the soils, originating a dry matter production about 50% of the inoculated control. The introduction of annual medics in these pastures requires the inoculation of the seeds with efficient rhizobial strains.
Statistical analyses
Principal component analysis
A principal component analysis based on 16 variables (physical, chemical, biological and pasture yield) from 40 soils, sampled on 13 farms was made (Figure 1).
Figure 1 - Projections of the 40 soil samples onto the plane defined by the first and the second principal axes with minimum spanning tree superimposed. The ellipses enclose the soil samples with similar properties
The first three principal components (PC) account for 60.4% (PC1 = 35.4%; PC2 = 17.7%; PC3 = 7.3%) of the total variation in the original matrix. In spite of this relatively low value; the original distances had been preserved since the cophenetic correlation between the matrix distances implied in the PCA and the original ones was 0.90. The projections of the 16 variables onto the plane defined by the principal axes (I and II) are presented in the Figure 2. The correlations between the 16 original variables and the 3 principal components (Table 2) showed the individual contribution of each variable (Figure 2) to the spatial distribution of the soil samples presented in Figure 1.
Table 2 - Correlation between the original properties and principal components
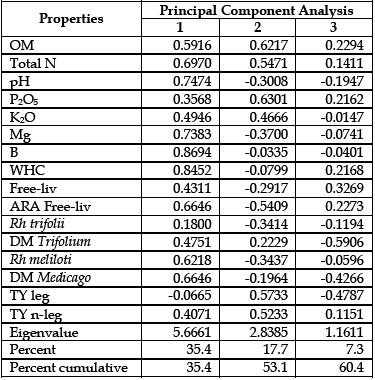
Figure 2 - Projections of the 16 soil properties used to characterize the 40 soil samples onto the plane defined by the first and the second principal axes
The PC1 separated the soil samples with higher values for the variables: Total N, pH, K2O, Mg, B, WHC, Free-liv, ARA Free-liv, Rh meliloti and DM Medicago, on right side of Figure 1, from the soil samples with lower values for the same variables. The PC2 separated the soil samples with higher values for the variables OM, P2O5, and TY (leg and non-leg), on upper side of Figure 1, from the soil samples with lower values for the same variables. The PC3 (not shown) separated the soil samples with higher values for the variable DM Trifolium from the soil samples with lower values for this variable (Table 2).
In Figure 1, we can verify the existence of 4 groups of soil samples and 2 outliers (gr 31, a very poor soil with low values for almost all variables and gr 34 a rich soil for almost all variables). The group A includes the soil samples: sch 8, sch 15, sch 28, gr 19, with higher values for the variables P2O5, OM, K2O total N and TY leg and non-leg. The group B includes the soil samples: sch 26, and sch 27 with higher values for almost all biological variables and some chemical and physical variables (B, WHC, Mg and pH). The group C includes the soil samples: sch 1, sch 2, sch 3, sch 5, sch 7, sch 10, sch 12, sch 13, sch 14, sch 25, gr 17, and gr 18, with intermediate values for almost all biological variables and for pH, Mg, B and WHC. The group D includes the remaining soil samples with lower values for biological variables. The obtained groups of similarity join soil samples with similar geological origin.
In Figure 2, we can verify the importance of each variable in the spatial distribution of soil samples (Figure 1) and to observe that some chemical variables, mainly OM, P2O5, K2O and total N seem to be related to legume and non-legume pasture yield.
Discriminant analysis
The discriminant analysis used to verify the existence of a geographical pattern of soil samples distribution, showed accuracy of 91.9% in the separation of the different sampling locals (Figure 3).
Figure 3 - Discriminant analysis of the soil samples geographical origin with the properties vectors superimposed
The first discriminant function accounted for 58.1% of the variance, discriminating the soil samples from different locals, being Nisa on the upper left side and Serpa and Portalegre on the down right side of the figure. It represents the contrast of the variables B, Mg, pH, Rh meliloti, DM Medicago and ARA Free-liv, with high levels at Serpa and Portalegre and low levels at Nisa. The second discriminant function accounted for 12.7% of the variance and allowed the individualization of the remaining sampling locals, representing the contrast between the two variables TY n-leg and P2O5 against K2O, Total N and OM, being the soil samples positioned according to the values of these variables. These results can be used as a complement of the PCA providing information on which variables are best adequate for discriminating the sampling locals.
The soil samples from each local were considered as uncorrelated soil samples. This methodology seems to be adequate, since the soil samples dispersion (Figure 3) in each local shows a general variability.
When the discriminant analysis was used to group the soil samples according to their geological origin (Figure 4), it was verified a good separation (89% accuracy) between the 2 origins, except for 2 misclassified soil samples, 1 schistose soil (sch 11) that is classified as granitic soil and 1 granitic soil (gr 18) that is classified as schistose soil. The soil sch 6 can be classified either as schistose (48%) or as granitic (52%). The soil samples sch 26 and sch 27 are a different group as already seen in PCA. The granite-derived soils, in general, have higher values for TY n-leg, being TY leg not important to discriminate the soils from different geological origin.
Figure 4 - Discriminant analysis of the soil samples according to their geological origin. The ellipses enclose the soil samples with the same geological origin, ▲- schist, · - granite (♦ sch 26 and ♦ sch 27 are outliers)
Correlations of soil physical, chemical and biological properties with yield are often low due to the interactions with weather, topography, and other biotic or abiotic factors. Associated to these constraints, the soil properties evaluated differ among the authors; the obtained results are frequently discrepant and cannot be applied without critical adjustments. Studies related to soil properties and herbaceous vegetation yield in the Mediterranean area are scarce. REZAEI et al., 2006, studying the relationships between soil chemical and physical properties and plant growth on the mountainous rangeland of northern Iran, a Mediterranean area, obtained results indicating that plant variables were more sensitive to soil physical properties than to soil chemical properties. Our results are different, showing that chemical properties are key factors to pasture yield.
Conclusions
Soils chemical and physical properties in the "montado" ecosystem are acceptable having low values for pH and P2O5. Properties related to number and quality of free-living nitrogen fixing bacteria and rhizobial populations associated to subclovers are adequate, contrasting with properties of rhizobial populations associated to annual medics, that are insufficient for a good nodulation, having a low nitrogen fixing potential.
With the principal component analysis it were obtained groups of similarity, being the schistose soils dominant in the groups A, B and C and the granitic soils dominant in the group D. The groups are dispersed over all the study area, suggesting that soil properties are independent of the soil samples and that some chemical properties are important factors to pasture yield.
The geographical distribution of the soil samples, when using the discriminant analysis, showed a large variability even in soil samples collected within a short distance. A good separation of the soil samples according to their geological origin was verified, being the granitic soils more productive in non-legume pasture.
Acknowledgements
This research was founded by the PARIPIPI-E
References
BROCKWELL, J., 1981. A strategy for legume nodulation research in developing regions of the world. Plant and Soil 58: 367-382. [ Links ]
BROCKWELL, J., HOLLIDAY A.R., PILKA, A., 1988. Evaluation of the symbiotic nitrogen-fixing potential of soils by direct microbiological means. Plant Soil 108: 163-170. [ Links ]
CHOTE, J., SCHWATZAMANN, A., BALLY, R., MONROZIER, L.J., 2002. Changes in bacterial communities and Azospirillum diversity in soil fractions of a tropical soil under 3 or 19 years of natural fallow. Soil Biology & Biochemistry 34: 1083-1092. [ Links ]
DOMMERGUES, Y., BALANDREAU, J., RINAUDO, G., WEINHARD, P., 1973. Non-symbiotic nitrogen fixation in the rhizospheres of rice, maize and different tropical grasses. Soil Biology & Biochemistry 5: 87-92. [ Links ]
GARCIA, C., ROLDAN, A., HERNANDEZ, T., 1997. Changes in microbial activity after abandonment of cultivation in a semiarid Mediterranean environment. JournalofEnvironmental Quality 26: 285-291. [ Links ]
HARDY, R.W.F., BURNS, R.C., HOLSTEN, R.D., 1973. Application of the acetylene reduction assay for the measurement of nitrogen fixation. Soil Biology & Biochemistry 5: 47-81. [ Links ]
HERRIDGE, D., GEMEL, G., HARTLEY, E., 2002. Legume inoculants and quality control. In Inoculants and Nitrogen Fixation of Legumes in Vietnam.ACIAR Proceedings No.109e, Herridge, D., NSW, Australia, pp. 105-115.
HINTZE, J., 2001. NCSS and PASS. Number Cruncher Statistical Systems. Kaysville, Utah.
JOFFRE, R., VACHER, J., LLANOS, C., LONG, G., 1988. The dehesa: an agrosilvopastoral system of the Mediterranean region with special reference to the Sierra Morena area of Spain. Agroforestry Systems 6: 71-96. [ Links ]
KAVADIA, A., VAYENAS, D.V., PAVLOU, S., AGGELIS, G., 2007. Dynamics of free-living nitrogen-fixing bacterial populations in antagonistic conditions. Ecological Modelling 200: 243-253. [ Links ]
LABORATÓRIO QUÍMICO AGRÍCOLA REBELO DA SILVA, 2000. Manual de Fertilização das Culturas. INIA, LQRS, Lisboa, 221 pp.
LOURENÇO, M.E., GONÇALVES, M.C., OLIVEIRA, A.J., SERRANO, J.E., 1994. Capacidade produtiva das pastagens naturais nas condições do montado alentejano. Pastagens e Forragens 14/15: 139-148. [ Links ]
MORENO, G., 2008. Response of understorey forage to multiple tree effects in Iberian dehesas. Agricult Ecosys Environ 123: 239-244. MORENO, G., OBRADOR, J.J., GARCIA, A. 2007. Impact of evergreen oaks on soil fertility and crop production in intercropped dehesas. Agriculture, Ecosystems & Environment 119: 270-280. [ Links ] [ Links ]
MORRIS, D.R., ZUBERER, D.A., WEAVER, R.W., 1985. Nitrogen fixation by intact grass soil cores using nitrogen 15 and acetylene reduction. Soil Biology & Biochemistry 17(1): 87-92. [ Links ]
OLEA, L., SAN MIGUEL-AYANS, A., 2006. The Spanish dehesa. A traditional Mediterranean silvopastoral system linking production and nature conservation. In 21st General Meeting of the European Grassland Federation: Sustainable Grassland Productivity Lloveras, J., González-Rodríguez, A., Vázquez-Yañez, O., Piñeiro, J., Santamaría, O., Olea, L., Poblaciones, M.J., Badajoz, pp 1-15.
OLIVEIRA, A., PAMPULHA, M.E., 2006. Effects of long-term heavy metal contamination on soil microbial characteristics. Journal of Bioscience and Bioengineering 102: 157-161. [ Links ]
PARKINSON, D., GRAY, T.R.G., WILLIAMS, S.T., 1971. Methods for Studying the Ecology of Soil Micro-organisms. IBP Handbook No 19. Blackwell Scientific Publications, Oxford, 351 pp.
PARREIRA, J.S., GARRIDO, R.M.I., 1987. Contributo para o estudo de implantação de pastagens de sequeiro no Baixo Alentejo. Pastagens e Forragens 8(2): 129-140. [ Links ]
POCHON, J., TCHAN, Y.T., 1948. Précis de Microbiologie du Sol. Monographie de l'Institut Pasteur. Masson et Cie, Paris.
QUIGLEY, P.E., CUNNINGHAM, P.J., HANNAH, M., WARD, G.N., MORGAN, T., 1997. Symbiotic effectiveness of Rhizobium leguminosarum bv. trifolii collected from pastures in south-western Victoria. Australian Journal of Experimental Agriculture 37(6): 623-630. [ Links ]
RODRÍGUEZ-ZARAGOZA, S., GONZÁLEZ-RUÍZ, T., GONZÁLEZ-LOZANO, E., LOZADA-ROJAS, A., MAYZLISH-GATI, E., STEINBERGER, Y., 2008. Vertical distribution of microbial communities under the canopy of two legume bushes in the Tehuacán Desert, Mexico. European Journal of Soil Biology 44: 373-380. [ Links ]
REZAEI, A.S., GILKES, J.R., ANDREWS, S.S., 2006. A minimum data set for assessing soil quality in rangelands. Geoderma 136: 229-234. [ Links ]
ROHLF, F,J., 2000. NTSYS-pc Numerical Taxonomy and Multivariate Analysis System. Version 2.1. Exeter Publications, Setauket, New York.
SLATTERY, J.F., COVENTRY, D.R., 1993. Variation of soil populations of Rhizobium leguminosarum bv. trifolii and the occurrence of inoculant rhizobia in nodules of subterranean clover after pasture renovation in north-eastern Victoria. Soil Biology & Biochemistry 25: 1725-1730. [ Links ]
SLATTERY, J., PEARCE, D., 2002. The impact of background rhizobial populations on inoculations response. In: Inoculants and Nitrogen Fixation of Legumes in Vietnam. ACIAR Proceedings No.109e, Herridge, D., NSW, Australia, pp. 37-45.
SOARES, R.S., ROESCH, L.F.W., ZANATTA, G., CAMARGO, F.A.O., PASSAGLIA, L.M.P. 2006. Occurrence and distribution of nitrogen fixing bacterial community associated with oat (Avena sativa) assessed by molecular and microbiological techniques. Applied Soil Ecology 33: 221-234. [ Links ]
THOMPSON, J.A., GEMELL, L.G., ROUGHLEY, R.J., EVANS, J., NICHOLLS, P.J., 1984. Nitrogenase activity associated with pasture grasses in Northern New South Wales. Soil Biology & Biochemistry 16: 217-222. [ Links ]
TRUJILLO, R.G., MATA, C., 2001. The Dehesa: an extensive livestock system in the Iberian Peninsula. In: Proceedings of the Second NAHWOA Workshop. Diversity of Livestock Systems and Definition of Animal Welfare. Hovi, M., Garcia Trujillo, R., University of Reading, UK, pp 50-61.
VINCENT, J,M., 1970. A Manual for the Practical Study of Root-nodule Bacteria. IBP Handbook No 15. Blackwell Scientific Publications, Oxford, 164 pp.
XIE, G., STEINBERGER, Y., 2002. Dynamics of the nitrogen-efficient guild and its relationship to nitrogen and carbon patterns in two desert soil ecosystems. Arid Land Research and Management 16: 69-81. [ Links ]
WEIER, K.L., 1980. Nitrogenase activity associated with three tropical grasses growing in undisturbed soil cores. Soil Biology & Biochemistry 12: 131-136. [ Links ]
WRIGHT, S.F., WEAVER, R.W., 1981. Enumeration and identification of nitrogen-fixing bacteria from forage grass roots. Applied and Environmental Microbiology 42: 97-101. [ Links ]
Entregue para publicação em Setembro de 2010
Aceite para publicação em Outubro de 2010













