Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Silva Lusitana
versão impressa ISSN 0870-6352
Silva Lus. vol.19 n.Especial Lisboa 2011
Susceptibility Variation in Eucalyptus spp. in Relation to Leptocybe invasa and Ophelimus maskelli (Hymenoptera: Eulophidae), Two Invasive Gall Wasps Occurring in Portugal
1 Durand, N., 1 Rodrigues, J.C., 2 Mateus, E., 3 Boavida, C. and 1 Branco, M.
1 Centro de Estudos Florestais. Instituto Superior de Agronomia. Universidade Técnica de Lisboa. Tapada da Ajuda, 1349-017 LISBOA
2 GUECKO. Departamento de Ciências e Engenharia do Ambiente. FCT, Universidade Nova de Lisboa, 2829-516 CAPARICA
3 Instituto Nacional dos Recursos Biológicos. MAMAOT, Edifício 1, Tapada da Ajuda, 1349-017 LISBOA
Abstract
Leptocybe invasa (Fisher & LaSalle) and Ophelimus maskelli (Ashmead) are two new invasive pests, originating from Australia, which are spreading quickly in the Mediterranean countries, Africa, the Middle East and Asia. Leptocybe invasa causes typical bump-shaped galls on the leaf midribs, petioles and stems of new growth of several Eucalyptus species and O. maskelli induces small round galls on both adaxial and abaxial leaf surfaces. Field sampling was conducted with the aim to determine differences in susceptibility between E. camaldulensis and E. globulus, two host species of economic importance frequent in Portugal. Differences in susceptibility to L. invasa were found for the two species, a hybrid population and between families of Eucalyptus globulus ssp. globulus. Results demonstrated interspecific and intraspecific susceptibility variations. In order to understand the intraspecific variations, physical and chemical traits of the most susceptible and resistant genotypes were analyzed further. Physical analyses of Eucalyptus leaves by thickness measurements and leaf surface observations by SEM did not reveal significant differences between susceptible and resistant genotypes. On the contrary, chemical analyses concerning chemical fingerprints by NIRS and leaf volatiles by GC-MS revealed significant differences.
Key words: Plant susceptibility; gall wasps; Eulophidae; Eucalyptus
Variação da Suscetibilidade de Espécies de Eucalyptus spp. em Relação a Leptocybe invasa e Ophelimus maskelli (Hymenoptera: Eulophidae), Duas Espécies Galícolas Invasoras em Portugal
Sumário.
Leptocybe invasa (Fisher & LaSalle) e Ophelimus maskelli (Ashmead) são duas espécies invasoras, originárias da Austrália, que estão a disseminar-se pela Europa mediterrânica, África, Médio Oriente e Ásia. Leptocybe invasa provoca galhas típicas com forma de inchaço nas nervuras foliares, pecíolos e caules dos ramos de várias espécies de Eucalyptus, enquanto O. maskelli origina pequenas galhas redondas tanto na parte adaxial como abaxial da superfície das folhas. Com o objetivos de determinar as diferenças na susceptibilidade entre E. camaldulensis e E. globulus, duas espécies frequentes em Portugal, foram efetuadas amostragens no terreno direcionadas para detetar estas duas pragas. Foram encontradas diferenças na suscetibilidade para as duas espécies de eucaliptos, para uma população híbrida e para famílias de Eucalyptus globulus ssp. globulus. Os resultados demonstram uma variação de suscetibilidade inter e intra específica. Para compreender a variação intra-específica, traços físicos e químicos dos genótipos mais suscetíveis e resistentes foram adicionalmente analisados. A análise física da espessura das folhas de Eucalyptus e observações com MEV da superfície foliar não detetaram diferenças significativas entre genótipos suscetíveis e resistentes. No entanto, análises químicas por NIRS-fingerprint e extração de voláteis foliares por GC-MS permitiram detetar diferenças significativas entre genótipos.
Palavras-chave: Suscetibilidade vegetal; insetos galícolas; Eulophidae; Eucalyptus
Introduction
Leptocybe invasa (Fisher & LaSalle) (Hymenoptera: Eulophidae) is a newly discovered gall-inducing wasp on Eucalyptus spp. This invasive species was discovered in 2000, outside its natural distribution area, in several countries in the Middle East, Europe and Africa (MENDEL et al., 2004). This is the only species inside the genus Leptocybe, which belongs to the sub-family Tetrastichinae. Originating from Australia, this species is an invasive pest which has quickly spread over the Middle East and Asia, Africa and the Mediterranean countries where Eucalyptus species occur. All species belonging to three sections of this genus (Exsertaria, Latoangulata and Maidenaria) were found to be suitable hosts, namely E. botryoides, E. bridgesiana, E. camaldulensis, E. globulus, E. gunii, E. grandis, E. robusta, E. saligna, E. tereticornis and E. viminalis.
A second invasive Australian Eucalyptus gall wasp, Ophelimus maskelli (Ashmead) (Hymenoptera: Eulophidae), also appeared recently in the Middle East and Mediterranean countries and is also spreading quickly in this area. The two species have several particular biological and ecological characteristic traits in common, like multivoltinous develop-ment, thelytokous parthenogenesis and host species range. The large host range, common to both species, is unique within gall-inducing insects and raises interesting research questions in order to understand how gall insects interact with their host plants. In Portugal, this species was first detected in 2006 (BRANCO et al., submitted). The biological and ecological traits listed above may partly explain the success of L. invasa and O. maskelli, yet the lack of specific predators or parasites and the abundance of host trees also accounts for their success in the invaded regions. In their natural homeland, the two species were undetected until now, probably due to natural regulation.
Eucalyptusspp. are very important trees in low-altitude arid and semiarid lands of the Middle East and North Africa. Moreover, in numerous countries these exotic trees are planted with economic objectives, in particular for pulp or biomass production, as they are fast-growing trees with short revolution periods. At present, Eucalyptus globulus is the most important pulpwood species in temperate regions of the world, especially in the Iberian Peninsula, South America and South Africa whereas Eucalyptus camaldulensis (Dehnh.) is the most economically important planted hardwood species in the Middle East (MENDEL et al., 2004). In Portugal, E. camaldulensis does not have the same importance as E. globulus but is frequently planted in recreational areas and as a windbreak and alignment tree.
In this preliminary work, we aimed at determining differences in host plant susceptibility to L. invasa and O. maskelli between different Eucalyptus genotypes, considering both interspecific and intraspecific levels. As a second step, we analyzed physical and chemical leaf traits from susceptible and resistant genotypes which might be related to host plant / gall wasp interactions. Research was carried out in Portugal between September 2006 and August 2007.
Material and methods
Determination of sensitivity to L. invasa and O. maskelli in E. camaldulensis and E. globulus
Field observations regarding the susceptibility of Eucalyptus camaldulensis (Denhn.) and Eucalyptus globulus (Labill.) to the two gallers were made in three Portuguese locations where L. invasa and O. maskelli were observed and adult trees of these two Eucalyptus species were found in the vicinity. These were Almeirim (northeast of Lisbon), Estremoz (Central-Eastern Portugal) and Setubal (southeast of Lisbon). The choice of the host species is based on the fact that these are the two most common and most important Eucalyptus species cultivated in Portugal. In the first two locations, presence of L. invasa was previously recorded in 2003 (BRANCO et al., 2006). In the third location, presence of the insect was recorded for the first time and the other Eucalyptus gall wasp, O. maskelli, was found with it. On each site, crown samples were taken from eight adult trees of each species. Observations were made on 12 randomly selected branches, six in the lower crown and six in the upper crown. All the leaves on each branch were observed. Regarding attack by L. invasa, trees were classified in four general categories: 0-"without gall sign"; 1-"with few galls (under 10% of all leaves)"; 2-"with a moderate attack (between 10 and 50% of leaves attacked)"; 3-"with an intense attack (more than 50% of leaves attacked)". Concerning the attack by O. maskelli, branches were ranked in two categories: 0-"without gall sign"; 1-"with galls".
Determination of sensitivity variation between Eucalyptus hybrids to L. invasa
This study aimed to examine potential variations of susceptibility between different Eucalyptus hybrids to the gall-inducing wasp Leptocybe invasa. The study was carried out at the RAIZ research centre (Pegões, southeast of Lisbon). Attacks were reported of L. invasa on several trees inside a park of Eucalyptus hybrid clones. The population was composed of 1806 young trees resulting from 136 different crossings between 13 Eucalyptus species. A first sampling selection was made for crossings with at least five plant replicates. Secondly, special interest was attributed to hybrids with one Eucalyptus globulus parent, so 23 crossings were selected with one E. globulus parent. Final samplings comprised 301 trees, resulting from 74 crossings. On each tree, six branches of the upper crown were randomly selected and the total number of leaves and number of attacked leaves were counted.
Determination of intraspecific variation related to L. invasa within E. globulus families
This study was carried out at the same RAIZ research centre. Observations were made inside a park with several families of E. globulus ssp. globulus. All the trees composing the population were observed, for a total of 100 trees belonging to 63 genotypes. Trees were fully observed (lower, middle and upper crown) and ranked in four different categories of sensitivity corresponding to the total number of leaves with developed galls observed on each tree: i) "0% of all leaves attacked"; ii) "less than 1% of all leaves attacked"; iii) "less than 5% of all leaves attacked"; and iv) "more than 20% of all leaves attacked".
Leaf thickness measurement by optical microscopy observation
Leaf thickness was measured by optical microscope observation from two different genotypes of E. globulus ssp. globulus from the Pegões Park. Sampling was done once at the end of February and then in early May. Two leaves were collected from each tree, three trees being sampled per genotype. Leaves were stored inside thermal polyethylene bags with ice until their arrival at the laboratory. In the laboratory, leaves were fixed into glycerol (40% aqueous) on Petri dishes. Three fine cuts per leaf were made on the first half, on the petiole side. Cuts were made with a scalpel and acquisitions and measurements were taken with appropriate software (Zeiss, AxioVision) on a Pentium PC connected to an optical microscope (Zeiss, Axioskop 2) with a digital camera (Zeiss, AxioCam). Three measurements were done on both sides of the midrib, at the same place as for potential oviposition sites by Leptocybe invasa. Leaf thickness measurement (in mm) was used for data analysis.
Scanning electron microscopy of leaf surface
Scanning Electron Microscopy (SEM) was used to analyze the structure and thickness of the leaf adaxial surface (cuticle and waxes). Young developing leaves, equivalent to those attacked, were collected on four E. globulus ssp.globulus trees; two trees from the genotype most attacked and two from a resistant genotype. Collected leaves were put into thermal polyethylene bags. Still in the field, each leaf was cut into eight square pieces of 5 mm, keeping the midrib section. Then, all samples were fixated with 1.5 ml of a 2.5% glutaraldehyde solution inside 3 ml plastic flasks during three hours at 4°C. In the laboratory, samples were transferred into a sodium phosphate buffer solution (0.1 M, pH 7.2) during 24 hours at 4°C. Samples were then washed three times in distilled water, each time during half an hour. Dehydration was done using an ethanol gradient (30, 50, 70 and 95%). Leaf samples were soaked during one hour at each ethanol gradient and finally were put into absolute alcohol. The CO2 critical point dried was achieved in a Polaron BioRad E3500 and samples were coated with gold in a Jeol JFC-1200. Observations were carried out at 15 KV, on a Jeol JSM-5200LV scanning electron microscope equipped with a direct image acquisition system and connected to a computer system.
Leaf volatiles analysis by Solid Phase Micro Extraction-Gas Chromatography
Four E. globulus ssp. globulus were sampled by taking three mature, developed leaves on two trees from the most sensible genotype and on two trees from a resistant genotype planted just in the next row. After collection, samples were stored in liquid nitrogen until arrival at the laboratory where they were put in a freezer at -80°C. The first near-midrib halves of the leaves were cut into small pieces, including some midrib parts, to finally obtain a weight of 200 mg. Then leaf pieces were transferred into 7.0 ml sealed vials (Supelco, Bellefonte, USA). Their volatile fraction was extracted by solid-phase micro extraction using a 100 mm polydimethylsiloxane (PDMS) coated fibre. The headspace extraction was done at room temperature for 45 min and then fibre was introduced into the injection port. Analysis of the volatile compounds was performed by gas chromatography, using an HP 5890A gas chromatograph (Hewlett Packard, USA) equipped with flame ionization detection (GC-FID). The separation was achieved on a DB-5 capillary column with 30 m x 0.32 mm I.D., 1.0 mm thickness (J & W Scientific, Folsom, USA). Each analysis lasted 45 minutes, time enough to have all retention times for all leaf volatiles.
Chemical analysis by Near Infrared Spectroscopy (NIRS)
Ten mature developed leaves, analogues to those attacked, were collected on four randomly selected E. globulus ssp. globulus, two trees from each of the two genotypes between which the largest sensitivity variation was detected. Samples were put into thermal polyethylene bags and stored in ice. On arrival at the laboratory they were maintained at 4°C. Then six 5mm diameter discs were collected from each leaf. In total, 240 leaf disc samples were analyzed by NIRS, first fresh then after being dried for 48 hours at 60°C. A third analysis was conducted with the same samples, ground and dried again during 24 hours at 60°C. The two first analyses were done with a fibre probe (spectra from 10 000 to 5 100 cm-1 and resolution of 8 cm-1). The third analysis was done with an integration sphere (spectra from 10 000 to 4 000 cm-1 and resolution of 8 cm-1).
Statistical analyses
ANOVAs, Log-linear model analysis and Kruskal-Wallis tests were performed using SPSS (V.13 for Windows). Data concerning volatile compound concen-trations was treated with Principal Multivariate Analysis by using Canoco (V.4.5 for Windows). NIR statistical analyses were performed with Opus (Brucker, Germany) and Unscrambler (Camo, Norway, V.9 for Windows).
Results and discussion
Determination of sensitivity to L. invasa and O. maskelli in E. camaldulensis and E. globulus
Concerning L. invasa, the two host species, E. camaldulensis and E. globulus, were compared using a non-parametric Kruskal-Wallis test. In all localities, variation of the degree of attack was significant between the two species (a = 0.05). Figure 1 represents a box plot compiling data from three locations: Almeirim, Estremoz and Setubal. In Setubal, all E. camaldulensis were attacked to the same low degree (five percent of foliage attacked). The highest degree of attack was found in Almeirim, where in some trees more than half of the foliage was infested. In Estremoz, attack was intermediary. In the three locations, none E. globulus were attacked. Concerning O. maskelli, the percentage of infested leaves per branch was significantly higher for E. camaldulensis in comparison with E. globulus (F=12.9, df =1; 227, P<0.001).
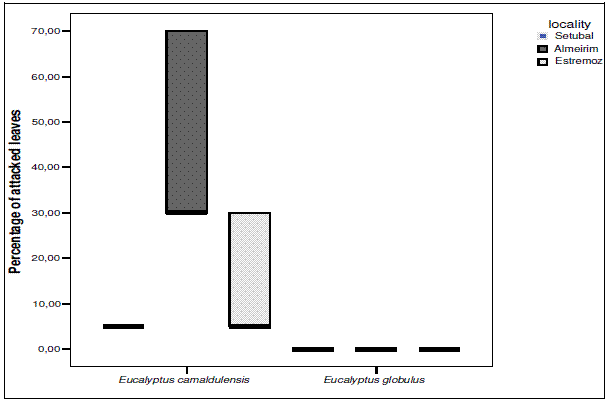
Figure 1 - Box plot showing percentage of attacked leaves by L. invasa for the two Eucalyptus species, E. camaldulensis and E. globulus, in Almeirim, Estremoz and Setubal
Host suitability to L. invasa and O. maskelli was tested in the laboratory by exposing seedlings of several Eucalyptus species and observing whether gall formation occurred (MENDEL et al., 2004; PROTASOV et al., 2007). We decided to study host suitability with populations of adult trees of two species, E. camaldulensis and E. globulus, occurring in the same natural environment. It is commonly known that sometimes differences in environmental conditions lead to different results. Previous research indicates that both of these species are susceptible to both L. invasa and O. maskelli but no work had been done on insect host plant preference. Results clearly show a difference of susceptibility relative to both L. invasa and O. maskelli between E. camaldulensis and E. globulus. E. camaldulensis was found to be much more susceptible than E. globulus, a result which concurs with previous preliminary field observations (BRANCO et al., 2006). This result can contribute to the determination of host plant preferences among susceptible host species. In Portugal, attacked E. globulus were only observed in two locations, one of them being the Eucalyptus park located near Pegões. In the other location the attacked tree was a single adult with few galls seen in its upper crown. All these observations reveal that E. globulus is mostly attacked at a very low level. On the contrary, both the field observations conducted in Portugal in this work and the results found in Israel by MENDEL et al. (2004) revealed that E. camaldulensis is a highly susceptible host plant for L. invasa. Susceptibility variations in E. camaldulensis between the three locations sampled can have variable origins, linked with plants, insects, environment or a mix of them. In Israel, galls were observed on both juvenile and mature E. globulus foliage but in Portugal, galls were only observed on mature leaves. Finally, it is possible to say that the host ranges of both L. invasa and O. maskelli display an interspecific variation in susceptibility within the possible host range, particularly visible between E. camaldulensis and E. globulus. It reveals that, even if this gall-inducing insect uses a large host range, preferences between species may exist and may influence insect behaviour.
Determination of sensitivity variation to L. invasa between Eucalyptus hybrids
Plant susceptibility was shown to vary between hybrid clones (Figure 2). Only seven clones were found completely free of galls. ANOVA revealed that the percentage of attacked leaves is significantly different between clones (F = 61.8 df = 73.13, P<0.01) and also between trees (F = 9.3, df = 1, P<0.01). Hybrids with E. globulus ssp. globulus as one parent were found in the three defined categories (Figure 2) but were particularly found in high numbers in the un-attacked trees. Using the number of clones with E. globulus ssp. globulus parents and the others in a contingency table, a log-linear model was applied. The best model to fit the data showed differences between categories but no interactions between categories and E. globulus parents. The model predicted about 34% of E. globulus ssp. globulus in each category, meaning that this parent species seems to be equally represented in each category (X2 = 1.3, df = 2, P = 0.53).
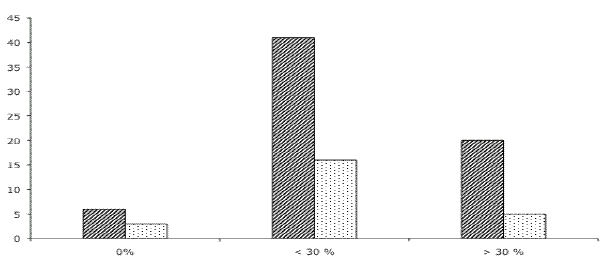
Figure 2 - Distribution of hybrid clones in three susceptibility classes: 0% of attacked leaves; less than 30% of attacked leaves; more than 30% of attacked leaves. Bars with oblique lines: total number of hybrid clones in each category. Bars with dots: number of hybrid clones with one parent being E. globulus ssp. globulus. Y axis: number of trees
Previous work has been done regarding Eucalyptus hybrids and insect herbivory, especially galling insects, tending toward conclusion of a higher sensitivity of hybrids to insects when they are compared with their parents, supporting both higher insect species richness and higher densities of insects (MORROW et al., 1994; DUNGEY et al., 2000). These results were extended with the hybrid bridge hypothesis (FLOATE and WHITHAM, 1993), suggesting that hybrid intermediates facilitate expansion of the host range to include previously unused species. In this experiment, we did not evaluate this hypothesis. Nevertheless, when we compared general galling density in hybrids with that in E. globulus ssp. globulus, present in a park of families only one hundred meters away, we found that the latter had a gall density much lower than the one found in hybrids with an E. globulus parent, agreeing with the hybrid bridge hypothesis. However, these comparisons are relative, only staying at the species level, because trees from the E. globulus ssp. globulus stand and the hybrids are not parentally related.
All parent species (the mother and father species of all hybrids) were identified. In the 13 species crossed, nine are strictly different species, two are different subspecies within a species (E. globulus ssp. maidenii and E. globulus ssp. globulus) and two are hybrid species: E. algeriensis (hybrid between E. camaldulensis and E. rudis) and E. trabutii (hybrid between E. camaldulensis and E. botryoides). All 13 parent species come from three sections of the genus: Exsertaria, Latoangulata and Maidenaria. Eight parent species out of the 13 were already tested and considered to be suitable hosts (MENDEL et al., 2004). Suitability of the five other species remains untested but they belong to the same three sections where the eight susceptible species are located. Results in this work showed that few hybrid genotypes were free of galls. Only seven out of 74 genotypes were not attacked at all. It is interesting to note that in all seven of these crossings, the father species was either E. globulus ssp. globulus or E. globulus ssp. maidenii.
Determination of intraspecific variation within Eucalyptus globulus Labill. families
In the Pegões park Eucalyptus globulus population, only one, two or three trees represented each genotype. For statistical analyses, only data concerning genotypes with repetitions were taken into account, i.e. 27 genotypes (Figure 3). The Kruskal-Wallis non-parametric test revealed significant susceptibility variation in E. globulus ssp. globulus relative to L. invasa (X2= 56.3, P = 0.01). Using the Mann-Whitney U-Test, genotypes were classified in three groups according to their densities of attacked leaves (Figure 3).
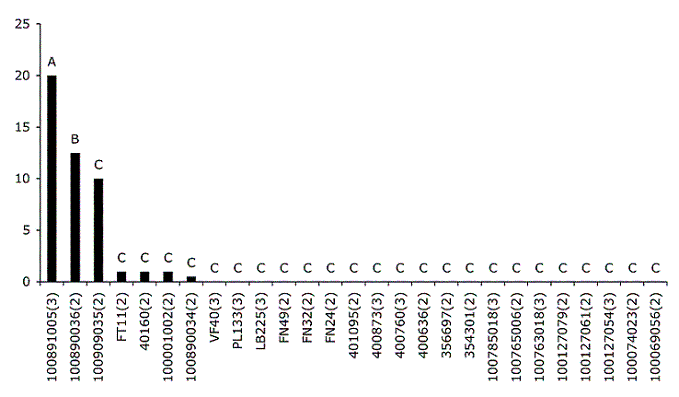
Figure 3 - Averaged percentage of attacked leaves for 27 E. globulus ssp. globulus genotypes. A, B, C represent the three statistically defined groups. Legends in abscissas represent genotype codes with (n): number of repetitions. Y axis: Percentage of attacked leaves
Eucalyptus globulus is a complex species composed of four subspecies defined by morphological differences (KIRKPATRICK, 1975). The forest industry has shown more interest in Eucalyptus globulus ssp. globulus (JONES et al., 2002) and this is the subspecies presently planted in Portugal for the pulp industry. Genetic variation has been observed for E. globulus in herbivory susceptibility of different geographically isolated races and families in relation to insects (JONES and POTTS, 2000) and in relation to mammals (O'REILLY-WAPSTRA et al., 2001). The present work focused on genetic variations in plant susceptibility among E. globulus ssp. globulus families. Our results showed significant susceptibility variations in relation to L. invasa, linked to plant genotypes. Consistent with the results obtained for the interspecific variation analysis, the observation of E. globulus ssp. globulus families revealed that only a few genotypes were massively attacked, while most of them were completely free of galls or not attacked much.
Leaf thickness measurement by optical microscopy observation
The resistant genotype of Eucalyptus globulus tends to have thicker leaves but the differences were not significant (F = 1.4, df = 1.68, P > 0.05). Leaf thickness can be an important factor in influencing insect herbivory. Different histological reasons may encourage plants to produce thicker leaves. The epidermis and cuticle layer (with waxes) can be important barriers and could stop insect oviposition. Inside the leaf, other tissues like sclerenchyma or mesenchyma can be denser. Thicker leaves can be a bigger resource for insects but can also be more protected from them. Moreover, leaf toughness can be affected, influencing the entrance of insect ovipositors inside tissues. In Eucalyptus spp., leaf thickness and particularly cuticle thickness increase with tree age (ENGLAND and ATTIWILL, 2005). In this study, results suggest that resistant leaves tend to be thicker, yet we found no significant statistical differences between leaf thicknesses of leaves from sensitive and resistant genotypes. For further analyses of leaf thickness, samples frozen in liquid nitrogen and cuts made by microtome could maybe give more precise thickness values.
Scanning electron microscopy of leaf surfaces
Observation of the adaxial surface of young leaves from both susceptible and resistant genotypes did not reveal any relevant difference. The surface cuticle is almost smooth, with small bumps accompanying the epidermal cells that originate an undulated surface. This also implies an irregularity in thickness. Cuticle thickness is 0.3 mm in the thicker elevated parts. At this stage in development, stomata are under the cuticle, without contact with the outside environment because few stomata pores are seen. Observations also revealed that developing young leaves already have important oil glands spread through the palisade mesophyll, near small conductor veins.
In plant – insect interactions, it is commonly known that leaf surface structure is an important factor in conditioning herbivory. Leaf surface is the first physical contact when insects arrive on a plant. It is also the first barrier built by the plant to protect underlying tissues. Some plants produce specific structures on the leaf surface like trichomes and hairs that undervalue insect action. Our preliminary study revealed no differences, for two types of Eucalyptus leaves (young, developing, and mature, developed) in leaf surface structure and cuticle thickness between the two genotypes. We conclude that the observed Eucalyptus leaves displayed xeromorphic traits like palisade mesophyll, thick cuticle and protected stomata. Comparing genotypes inside E. globulus ssp. globulus, we expected probable small differences in phenotypical traits such as leaf surface and cuticle thickness, yet our results did not reveal differences between the genotypes analyzed.
Leaf volatiles analysis by Solid Phase Micro Extraction - Gas Chromatography
Fourteen compounds were separated by GC-MS from volatiles extracted from the Eucalyptus leaves. A PCA applied to the data revealed a good separation in terms of the leaf volatile content between the sensitive and resistant genotypes along the first axis (Figure 4). Effectively, almost all samples from the sensitive genotypes are on the left side of axis 1 while all samples from the resistant genotype are on the right side. The main volatiles separating genotypes are green leaf volatiles (C6 alcohol or aldehyde), monoterpenes and sesquiterpenes. Green leaf volatiles and sesquiterpenes were most related with sensitive genotypes. On the contrary, monoterpenes, in particular a-pinene, limonene and 1,4-cineole, were most related with resistant genotypes. The largest concentrations among volatile compounds were those of a-pinene and 1,4-cineole.
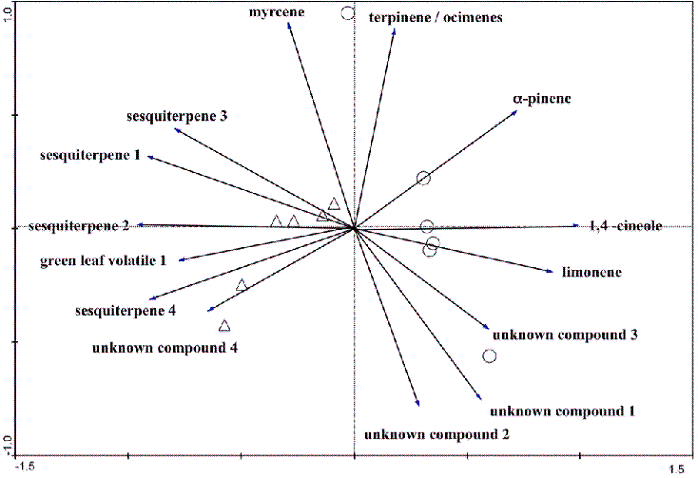
Figure 4 - PC analysis of leaf samples. Axes represent volatile compounds explaining more than 50% of the separation. Triangles represent samples from the sensitive genotype and circles, samples from the resistant genotype. Together, the two axes explain 82.4% of the separation
The complete system of host plant selection involves a three-link chain of events in which the first link is governed by cues from volatile plant chemicals, the central link by visual stimuli, and the final link by cues from non-volatile plant chemicals. Leaf volatiles can influence host recognition and selection by phytophagous insects, most of them being very susceptible to molecules emitted by trees. Further, plant volatiles may act as attractants or deterrent stimuli (for example non-host volatiles are known to have a deterrent effect on several insect species, allowing them to avoid non-host plants). In some cases, trees also emit compounds that attract predators or parasitoids of herbivorous insects. Emission of volatiles can have negative effects and in some other cases positive effects.
Volatile leaf oils were well studied in several Eucalyptus species, especially subgenus Monocalyptus and Symphyomyrtus (LI et al., 1996). For this second subgenus that incorporates E. globulus, composition of volatile leaf oils is poorly differentiated in the majority of species and these oils are rich in the monoterpenoids 1,8-cineole and a-pinene. This work confirms this second fact and also confirms previous assessments concerning genetic dependence of mono- and sesquiterpene biosynthesis and biogenesis. The present results support the hypothesis that intraspecific susceptibility variations in E. globulus ssp. globulus can be related to differences in leaf volatile concentrations, since we found differences in volatiles in the two different genotypes, which could be well separated by multivariate analysis. Furthermore, the results gave evidence that sensitive genotypes were more associated with the emission of green leaf volatiles and sesquiterpenes while resistant genotypes were more associated with higher emissions of monoterpenes. Other work is now required to see if observed differences in volatiles can be related to insect herbivory. Wind tunnels could be used in order to see how far volatiles are involved in host plant recognition. The employed technique could also be extended at the plant level to see if volatile differentiations increase. Plant parts suitable to L. invasa including buds, young leaves and stems may be isolated and volatiles emitted by these parts could be extracted and analyzed by solid-phase micro-extraction gas chromatography.
Chemical analysis by Near Infrared Spectroscopy (NIRS)
Spectra from fresh and dried leaves and milled dried samples were very different, mainly due to the presence of water bands in the spectra of fresh leaves, this being an important absorber of NIR light. The PCA results of all data sets (fresh and dried leaves and the milled dried material) show that it is possible to differentiate between susceptible and resistant genotypes based on those spectra. Higher differentiation was observed with dried samples where these were clearly separated in two clusters, each one including only samples from one genotype.
NIR spectra contain chemical information about the sample, providing its chemical fingerprint. There are few reports of leaf analyses by NIRS; however, it can be a very good tool for environmental bio-monitoring (GÄB et al., 2006). This work shows that this technique could be applied to find differences between leaves from E. globulus genotypes showing different susceptibility to Leptocybe invasa. Results clearly revealed a difference in the chemical fingerprint of leaves from the two genotypes. Unfortunately, interpretation of spectra is difficult, mainly because of the superimposition of chemical information concerning samples. In the present work, chemical differentiation between the two genotypes is optimum with spectra of dry intact leaves, probably due to the contribution of the hydrocarbons of the cuticles. A future orientation of research could be to isolate the cuticles from susceptible and resistant genotypes then check again using NIR spectra whether it is possible to differentiate them.
References
BRANCO, M., FRANCO, J.C., VALENTE, C., MENDEL, Z., 2006. Survey of Eucalyptus gall wasps (Hymenoptera: Eulophidae) in Portugal. Bol. San. Veg. 32: 199-202. [ Links ]
DUNGEY, H.S., POTTS, B.M., WHITHAM, T.G., LI, H-F., 2000. Plant genetics affects arthropod community richness and composition: evidence from a synthetic eucalypt hybrid population. Evolution 54(6): 1938-1946. [ Links ]
ENGLAND, J.R. ATTIWILL, P.M., 2005. Changes in leaf morphology and anatomy with tree age and height in the broadleaved evergreen species, Eucalyptus regnans F. Muell. Trees 20: 79-90. [ Links ]
FLOATE, K.D., KEARSLEY, M.J.C., WHITHAM, T.G., 1993. Elevated herbivory in plant hybrid zones: Chrysomela confluens, Populus and phenological sinks. Ecology 74(7): 2056-2065. [ Links ]
GÄB, M., HOFFMANN, K., LOBE, M., METZGER, R., OOYEN, S.V., ELBERS, G., KÖLLNER, B., 2006. NIR-spectroscopy investigation of foliage of ozone-stressed Fagus sylvatica trees. Journal of Forest Resources 11: 69-75. [ Links ]
JONES, T., POTTS, B.M., 2000. Genetic variation in susceptibility of Eucalyptus globulus coppice regrowth to shoot-feeding weevils (Myllorhinus spp.). Tasforest 12: 147-154. [ Links ]
JONES, T.H., POTTS, B.M., VAILLANCOURT, R.E., DAVIES, N.W., 2002. Genetic resistance of Eucalyptus globulus to autumn gum moth defoliation and the role of cuticular waxes. Can. J. For. Res. 32: 1961-1969. [ Links ]
LI, H., MADDEN, J.L., POTTS, B.M., 1996. Variation in volatile leaf oils of the Tasmanian Eucalyptus species II. Subgenus Symphyomyrtus. Biochemical Systematics and Ecology 24(6): 547-589. [ Links ]
KIRKPATRICK, J.B., 1975. Geographical variation in Eucalyptus globulus. Australian Government Publishing Service, Canberra. [ Links ]
MENDEL, Z., PROTASOV, A., FISHER, N., LA SALLE, J., 2004. Taxonomy and biology of Leptocybe invasa gen. & sp. n. (Hymenoptera: Eulophidae), an invasive gall inducer on Eucalyptus. Australian Journal of Entomology 43(2): 101-113. [ Links ]
MORROW, P.A., WHITHAM, T.G., POTTS, B.M., LADIGES, P., ASHTON, D.H., WILLIAMS, J.B., 1994. Gall-forming insects concentrate on hybrid phenotypes of Eucalyptus hosts. In The Ecology and Evolution of Gall-Forming Insects, pp 121-134. [ Links ]
O'REILLY-WAPSTRA, J.M., POTTS, B.M., McARTHUR, C., DAVIES, N.W., TILYARD, P., 2001. Inheritance of resistance to mammalian herbivores and of plant defensive chemistry in an Eucalyptus species. Journal of Chemical Ecology 31(2): 357-375. [ Links ]
PROTASOV A., LA SALLE, J., BLUMBERG, D., BRAND, D., SAPHIR, N., ASSAEL, F., FISHER, N., MENDEL, Z., 2007. Biology, Revised Taxonomy and Impact on Host Plants of Ophelimus maskelli, an Invasive Gall Inducer on Eucalyptus spp. in the Mediterranean Area. Phytoparasitica 35(1): 50-76. [ Links ]
Acknowledgments
We thank the RAIZ Research Institute for allowing us to work in their research stands, Ana Alves from ISA for the NIR spectroscopy, Pr. G. Teixeira (FFUL) and T. Nunes (FCUL) for their help with SEM. We also thank Pr. F. Lieutier (Université d'Orléans) for his support.













