Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Portuguesa de Saúde Pública
versão impressa ISSN 0870-9025
Rev. Port. Sau. Pub. vol.29 no.2 Lisboa jul. 2011
Methicillin–Resistant Staphylococcus Aureus (MRSA) in a Portuguese hospital and its risk perception by health care professionals
David Peresa, Elaine Pinab, Margarida Cardosoc
aInfection Control Unit, Hospital Pedro Hispano, Matosinhos, Portugal. Grupo Coordenador Regional de Controlo de Infecção, Administração Regional de Saúde do Norte, Porto, Portugal
bPrograma Nacional de Controlo de Infecção, Direcção–Geral da Saúde, Lisboa, Portugal
cICBAS – Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Porto, Portugal. CIIMAR – Centro Interdisciplinar de Investigação Marinha e Ambiental, Porto, Portugal. ISPUP – Instituto de Saúde Pública da Universidade do Porto, Porto, Portugal. mcard@icbas.up.pt
Abstract
Objective: To describe the epidemiology of methicillin–resistant Staphylococcus aureus (MRSA) and to assess its perception by healthcare professionals. Design: Survey, through a two–part questionnaire. Setting: A 441–bed district general hospital. Participants: Part I – Inpatients over 16 years of age, in whom a non–nasal MRSA was isolated between February and August of 2005. Part II – nurses and doctors responsible for these patients. Methods: Part I – Demographic and clinical data collected from medical notes. Part II – Perception of doctors and nurses. Observed agreement and "Kappa" statistic were used to compare perceptions. A P value lower than 0.05 was considered to be statistically significant. Results: Of the 111 patients identified, 50.9% had history of hospitalization during the previous year, with high exposure to antimicrobial therapy and invasive procedures. Hospital stay was 4.5 times higher than the average inpatients and mortality 5.5 times higher. Proportion of MRSA was 60.0%, with an incidence density of 1.66%. Although agreement between nurses and doctors was low, the majority admitted nosocomial origin of the MRSA and its transmission through the hands of professionals. Reinforcement of hand hygiene was considered important to manage these patients by 69.4% of nurses and 64.9% of doctors. Additionally, all nurses and 89.4% of doctors agreed on the need to isolate these patients. Conclusions: High endemic level of MRSA detected in a susceptible population, associated with a lower awareness of management of these patients by doctors, compared with nurses, justifies a global programme to control MRSA. This programme should include consensus–based measures for management of patients, rational use of antimicrobials, and dynamic and focused educational programmes.
Keywords: MRSA. Infection control. Risk perception. Portugal.
Staphylococcus Aureus Resistente à Meticilina (MRSA) num hospital distrital do Grande Porto e percepção do risco pelos profissionais de saúde
Resumo
Objectivo: Descrever e analisar a epidemiologia do Staphyloccocus aureus resistente à meticilina (MRSA) num hospital distrital de 441 camas do Grande Porto, bem como a percepção que enfermeiros e médicos têm do problema. Desenho do estudo: Estudo transversal descritivo, através da aplicação de um inquérito de duas partes. Definição: Um hospital distrital de 441 camas. Participantes: Parte I – doentes internados com mais de 16 anos em que foi detectado MRSA não nasal, entre Fevereiro e Agosto de 2005. Parte II – enfermeiros e médicos responsáveis pela prestação de cuidados aos referidos doentes. Métodos: Parte I – recolha de dados demográficos, clínicos e factores de risco dos processos dos doentes. Parte II – aplicação de um inquérito a enfermeiros e médicos para análise das suas percepções. O acordo observado e a estatística "Kappa" foram utilizados para comparar as respostas entre classes de profissionais. O nível de significância adoptado foi de 5%. Resultados: Dos 111 casos estudados, 50,9% tinham historial de internamentos até há um ano atrás e 83,8% haviam estado expostos a antibioticoterapia prévia. O tempo de internamento foi 4,5 vezes maior que a média da população internada neste hospital, e a mortalidade 5,5 vezes maior. A prevalência de MRSA foi de 60,0% e a densidade de incidência de 1,66 casos por mil dias de internamento. A grande maioria dos profissionais admite que o MRSA é adquirido no ambiente hospitalar e que são as mãos dos profissionais de saúde a principal via de transmissão. Como medidas para gerir doentes com MRSA, 69,4% dos enfermeiros e 64,9% dos médicos referem o reforço da higienização das mãos, a totalidade dos enfermeiros e 89,4% dos médicos concordam com a necessidade de algum tipo de medidas de isolamento. Conclusões: Constatou–se a existência de altos valores endémicos de MRSA. Os profissionais têm a percepção da associação do MRSA aos cuidados de saúde, bem como a importância das mãos dos profissionais como veículo de transmissão, no entanto a classe médica está menos sensibilizada para as medidas de gestão para estes doentes. Parece justificar–se um programa global para controlo deste microrganismo, na gestão de doentes colonizados ou infectados por MRSA, na utilização racional dos antibióticos, bem como a formação dos profissionais de saúde, com um carácter mais dinâmico e dirigido.
Palavras chave: MRSA. Controlo da infecção. Percepção do risco. Portugal.
Introduction
Staphylococcus aureus is a Gram-positive bacterium that colonizes the skin of about 30% of healthy humans, although mainly a harmless coloniser, S. aureus can cause severe infection. Its oxacillin-resistant form (Methicillin-Resistant S. aureus, MRSA) is the most important cause of antibiotic-resistant healthcare-associated infections worldwide.1 Of the expected 2 billion individuals carrying S. aureus worldwide, conservative estimates, based on either Dutch or United States of America (USA) prevalence figures, would predict that between 2 million and 53 million carry MRSA.2 There are several studies which conclude that nosocomial MRSA infection increases morbidity, mortality, length of stay and costs.3-7 Historically, MRSA isolates have been associated with nosocomial infections, however, in recent years, different strains with unique phenotypes have emerged in the community, and the reservoir of this community-associated MRSA (CA-MRSA) is rapidly expanding.8 Mathematical models have shown that CA-MRSA has a high potential to become endemic in the community, and this will impact significantly on the control of MRSA in the hospital setting.9
For healthcare facilities, surveillance is an important and approved method to assess the incidence of infection due to multidrug-resistant bacteria and to improve infection control measures, if necessary.10 Various protocols and guidelines have been developed in an attempt to prevent the development and slow down in the transmission of nosocomial MRSA. If healthcare professionals (HCP's) are to be successful in this objective, then understanding the epidemiology, pathogenesis and routes of transmission of MRSA in healthcare facilities becomes critically important.11 In this study we describe the epidemiologic characteristics of the MRSA in a Portuguese district general hospital, as well as its perception by the HCP's.
Methods
Study setting and infection control practices
This study was conducted at Pedro Hispano Hospital, a district general hospital in the north of Portugal with 441 beds. This facility, that serves a population of approximately 430,000, is part of a "local healthcare unit", which integrates the management of primary healthcare resources of the city of Matosinhos. Information about multirresistant microorganisms, including the MRSA (which is endemic in this facility) is available annually.
All patients with results positive for MRSA were treated with contact isolation measures, as recommended by the local infection control manual, based on the "Center for Disease Control and Prevention" (CDC) guidelines available at the time of the study.12 Intensive care patients of both existing units were screened for nasal colonization at admission and every 4 days and, if positive for MRSA, decolonization was attempted with topical agents (nasal mupirocin and chlorhexidine bathing).
Participants and data collection
Between February 21 and August 31, 2005 (192 days), the prospective infection surveillance system, based on laboratory results, identified all cultures positive for MRSA. Patients were excluded from the study if they: (a) were less than 16 years old, (b) had only nasal colonization detected, (c) had died or been discharged when the result was known or (d) were treated in ambulatory care. A two part questionnaire was applied to the 111 cases who met the criteria, after it was pretested and approved by the institutional ethics board.
In the first part, the investigators recovered demographic and clinical data from the medical record of each patient and, in the second part, the questionnaire was administered to the doctors and nurses responsible for this patient, in order to assess their perception of the epidemiology of MRSA (meaning of the strain identified, source and route of transmission) as well as the proper conduct to be followed to take care of this patient.
Definitions and terms
The risk factors associated with the use of indwelling devices (peripheral and central vascular catheters, urinary catheter, gastrointestinal tube, wound drainage device, endotracheal tube or tracheostoma and hemodialysis) were considered if they were present during the last 4 days, exposure to antibiotics during the last 7 days and, in case of a surgical procedure, during the last 30 days before the recovery of the sample which detected the MRSA.
"Immunodeficiency" was considered when the patient had any immunological disease or immunosuppressive therapy (chemotherapy, radiotherapy or steroids) in high doses during at least 15 days before the recovery of the sample which detected the MRSA.13
The terms "single-room isolation" (isolation of patients in a single-bedded room), "cohorting" (physical segregation of a group of patients with MRSA from the other patients in a geographically distinct area of the same ward) and "barrier nursing" (use of aprons or gowns, gloves and, in some cases masks, by the HCP as the only physical barrier to transmission) were used to refer to the level of isolation, as recommended by an extensive systematic review on isolation policies.14
"Morbidity" estimation was based on the indicators proposed by Ducel et al.15 and Horan and Gaynes16, MRSA rates, using three different denominators: proportion of MRSA (percentage of strains resistant to methicillin within the total of S. aureus identified), the incidence (number of MRSA per 1,000 admissions) and incidence density (number of MRSA per 1,000 patient-days).
Microbiological methods
Isolates were grown on blood and chocolate agar and confirmed as S. aureus by colony morphology, Gram stain, catalase test and through the rapid method "Staph aureus fumouze" (Fumouze Diagnostics, Paris, France). The antimicrobial susceptibility testing was determined by the automatic system "Vitek 2" (bioMérieux, Marcy l'Etoile, France). In case of doubt, confirmation of the minimum inhibitory concentration result was performed through "E-test" (AB Biodisk, Solna, Sweden). Strains with minimum inhibitory concentrations of 4 μg/mL of oxacillin or greater were considered resistant and 2 μg/mL or less, sensitive, according to the "Clinical and Laboratory Standards Institute" guidelines.17
Statistical analysis
Statistical analysis was performed using the "Statistical Package for the Social Sciences" (SPSS Inc., Chicago, USA) version 13.0. Categorical variables were compared using the chi-square or Fisher's exact test. To evaluate agreement between doctors and nurses, responsible for each patient, observed agreement and the "Kappa" statistic were applied. "Kappa" statistic was assessed to quantify the extent of agreement beyond chance achieved by the two groups of HCP's considered. The level of significance adopted was 5%.
Results
Demographic characteristics and risk factors
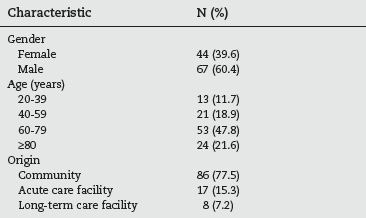
During the 192 day study period, 111 cases were detected. The demographic characteristics of these are listed in table 1. The male gender predominated (60.4% of the studied population) and almost half (47.8%) were between 60 and 79 years old. The majority of the patients came from the community (77.5%), but 17 of them (12.6%) were transferred from other hospitals and 8 (7.2%) from long-term care facilities.
Table 1 - Demographic characteristics of the studied population (n=111)
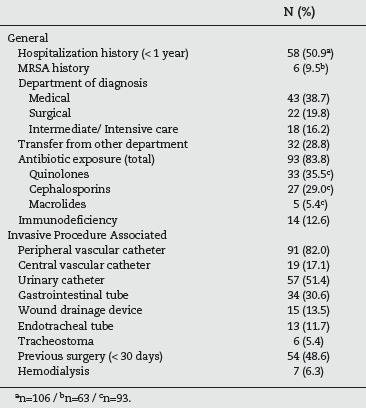
According to table 2, more than half (50.9%) of the patients had a history of hospitalization during the previous year and almost 10% had previously been detected with MRSA. The majority of cases were identified in medical specialities (38.7%) followed by surgical (19.8%) and intermediate/intensive care department (16.2%). However it should be taken into account that 28.8% of the patients had been transferred from another ward, before the one where the MRSA was identified. A vast majority (83.8%) were exposed to antimicrobial therapy during the week before, with quinolones in 35.5% of the cases and cephalosporins in 29.0%. With respect to the risk factors associated with invasive procedures, 82.0% had a peripheral venous catheter and 51.4% an urinary catheter. Almost half (48.6%) had been subjected to a surgical procedure in the last thirty days and 17.1% had a central vascular catheter.
Table 2 - Risk factors present in the studied population (n=111)
Morbidity and mortality
As listed in table 3, the mean length of stay was 40.7 days (median 31.5; range 5-182) with 20 patients (18.0%) who stayed more than 60 days.
Table 3 - Morbidity and mortality in the studied population: general and MRSA SPECIFIC (n=111)

More than one quarter of the studied population (27.0%) died in the hospital and 8 (7.2%) were transferred to other healthcare facilities. In 72.1% of the patients the MRSA was detected 48 hours after admission. The proportion of MRSA detected was 60.0%, the incidence was 11.9 per 1,000 admissions and incidence density revealed 1.66 cases per 1,000 patient-days.
Perception of the healthcare professionals
Table 4 shows the perception of the HCP's about the epidemiology of the MRSA, as well as the measures considered adequate to manage patients with positive cultures. More than 70% of the doctors and nurses considered the identified strains as hospital acquired infections, however, in the remaining cases, nurses referred twice as frequently as the doctors that the strains could be community acquired. For this question, the observed agreement was 74.2% and the Kappa value of 0.336, which corresponds to a "fair agreement" between observers.18
Table 4 - Perception of nurses and doctors of the epidemiology of MRSA and measures to manage positive patients
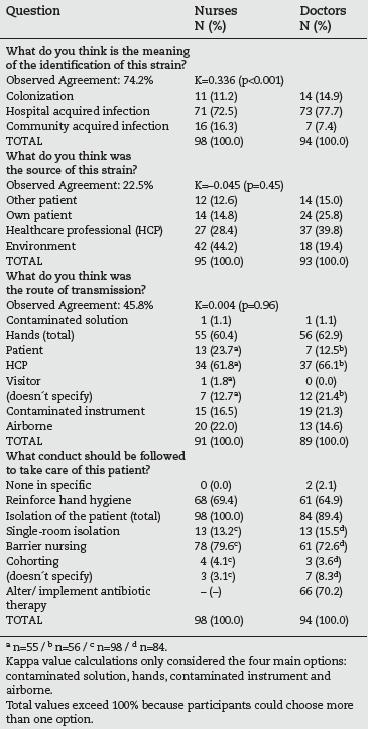
For the second question (perception about the source of the strain), 44.2% of the nurses answered the "environment", followed by 28.4% of these professionals who thought the source was a HCP. More doctors (39.8%) agreed to the hypothesis of the source being a HCP, 25.8% chose the option "own patient" and 18 doctors (19.4%) answered the "environment". For this question the observed agreement was low (22.5%) and the Kappa value (-0.045) compatible to "by chance" agreement between these two groups of professionals.
For the perception of the route of transmission, more than half of both nurses and doctors referred the hands as the main route (60.4 and 62.9%, respectively). Within this category, it was the "hands of a HCP" which was indicated most frequently (61.8% of nurses and 66.1% of the doctors). The second option was "airborne" for nurses (22.0%) and "contaminated instrument" for doctors (21.3%). The observed agreement was 45.8% and the Kappa value (0.004) was, again, compatible to "by chance" agreement.
When asked about the conduct to be followed for the care of MRSA positive patients, two (2.1%) doctors answered "none specifically". The response "reinforce hand hygiene" was chosen by almost 70% of the nurses and by 64.9% of the doctors. All the nurses (100%) and 89.4% of the doctors agreed that the patient needed some type of isolation. Within this category, the first option was "barrier nursing" (79.6% of the nurses and
72.6% of the doctors) followed by single-room isolation (13.3% and 15.5%, respectively). The need to alter/implement antibiotic therapy was assumed by 66 doctors (70.2%).
Discussion and conclusions
More than half of the studied sample had a history of hospitalization during the previous year and almost 10% had been previously diagnosed with MRSA, similarly to the 14.4% found by a study conducted in two terciary-care centers in Saudi Arabia.19
Interestingly, in the present study we found that it was in the medical department where most cases were detected, followed by surgical wards and intermediate/intensive care units, in contrast to other studies where the intensive care units (ICU) were in the first place.19-21 This difference could be explained by the aggressive strategy to control the MRSA in both ICU's of this hospital (we are not aware if this occurred in the other studies). This information should be interpreted carefully, considering the exclusion criteria of this study, namely the fact that patients who had died when the result was known were not studied, since this could have had an influence in the number of critical patients included. Other sources of bias could be the different ratios ICU/general beds in the hospitals studied and the mobility of the patients within the facilities: we identified that 28.8% of the patients had been in other wards before the one where the MRSA was identified. A Spanish study detected an even higher value: 37.8% in patients with bacteraemia by MRSA.22
Monnet et al.23 described several studies which conclude that exposure to cephalosporins, quinolones and macrolides constitutes risk factors for colonization or infection by MRSA. Similarly to our investigation, two other studies20,22 detected an exposure to antibiotics of over 70% in a population with MRSA and, in another hospital, around 66%.24 In contrast with the 35.5% described by us, a 640 bed USA hospital study25 identified an exposure of 67.6% of MRSA positive patients to quinolones.
With the respect to the risk factors associated with invasive procedures, Montesinos et al.20 likewise detected a similar proportion of patients with intravascular catheter (79%) and subjected to a previous surgical intervention (51%) but a higher exposure to urinary catheters (77%). In a Swiss study,24 45.7% of the MRSA colonized patients had a not specified indwelling device and 54.3% a history of surgery in the last thirty days.
The length of stay of the studied individuals was 4.5 times higher when compared to the general adult hospitalized population in this hospital (40.7 versus 9.0 days). This value was higher than the difference found in a USA study developed in an acute care military facility, where MRSA patients had a length of stay 3.3 times higher.26
Although the mortality value we described may not be directly related to the mortality caused by MRSA, it may be an indication of the morbidity associated with this microorganism, as well as the susceptibility of the population it affects. For this indicator a 5.5 fold difference was found between the studied population and the general adult hospitalized population (27.0% versus 4.9%). Madani et al.19 described a general mortality of 53.7% in a population infected with MRSA, but regarded as mortality directly associated with this infection 36.4% of the cases.
Usually an infection is considered acquired at a healthcare facility when it appears 48 hours after the admission.16 In this study 31 patients (27.9%) were identified as MRSA positive less than 48 hours after admission, which could lead us to consider a community origin. A Portuguese study27 involving 3,266 healthy individuals from the community detected low prevalence of MRSA nasal colonization (0.7%). However, all but 3 of our patients had classical risk factors for MRSA carriage: 6 came from other acute care facilities, 4 from long term care facilities and 18 had a history of hospitalization during the previous year.
MRSA rates in Portugal are know to be high: in 2005 the "European Antimicrobial Resistance Surveillance System" detected a proportion of resistance of 46.6% in Portugal,1 which is in agreement with the 47.5% of resistance reported in a Portuguese study involving 9 hospitals.28 Both values were still lower than the 60.0% detected in our study. A German study29 identified an average value of incidence density of 2.77%thou in 38 ICU's and a French multicenter study30 described a rise of this indicator from 0.71 to 0.96%thou between 1996 and 2000. Richet et al.31 studied 90 healthcare facilities around the world and detected median values of 0.40%thou for Western Europe and hospital category<500 beds. Once again the values found were lower than the one in our study (1.66%thou).
The majority of the HCP's had the perception that this MRSA strains were hospital acquired, but Kappa value identified just a "fair agreement" between nurses and doctors. A possible explanation for this would be the HCP's not taking into account the 48 hour timing generally used to distinguish hospital from community acquired infection. Considering the changing epidemiology of the MRSA, Klevens et al.32 proposed a different classification of cases into three mutually exclusive groups: (a) healthcare-associated community-onset; (b) healthcare-associated hospital-onset and (c) community-associated. This is an interesting approach and should be considered in future research in this area.
Nurses identified first the "environment" and then a "member of the staff" as the possible source (opposed to the doctors who chose "member of the staff" first), perhaps because nurses spend more time on the wards and recognize the limitations of the cleaning procedures. Both groups of HCP's are concerned about being themselves the source of the MRSA, more than the option "other patient". Bearing in mind that clinical microbiological cultures fail to identify up to 85% of MRSA-colonized patients,33 if the HCP has the perception that the other patients are not likely to be the source, this could have serious implications on the adherence to the Standard Precautions.
Slightly more doctors than nurses thought the route of transmission was the hands of a HCP. This is an interesting result considering that fewer doctors chose the option "reinforce hand hygiene" when asked about the management of MRSA positive patients. It would seem that there is a difference between the knowledge and importance of this practice and its day-to-day application. One study34 recognized that 62% of the doctors and 72% of nurses have the perception that they practice hand hygiene more than 80% of the times before and after contact with patients, concluding that HCP's perception of the compliance with infection control measures are better than their actual practice, as demonstrated by observational studies. CDC guidelines35 also indicate physician status as an observed risk factor for poor adherence to recommended hand-hygiene practices. It is interesting to see that a substantial number of HCP's identified the air as possible route of transmission (22% of the nurses and 14.6% of doctors). Although some studies36,37 suggest that MRSA is recirculated in the air, especially after movement, such as in bedmaking, it is considered that the transmission of MRSA within healthcare facilities primarily occurs via carriage on the hands of healthcare workers.11
The fact that two doctors and none of the nurses agreed that no specific measures were necessary to take care of MRSA positive patients and that 100% nurses identified the need to isolate these patients, in contrast with just 89.4% of the doctors, is in agreement with other studies. Afif et al.38 referred that the compliance to MRSA precautions is worse among doctors as compared to nurses (Odds Ratio 0.35; 95% confidence interval, 0.14 to 0.86). Seaton and Montazeri39 described, in a population of British doctors, that 10% did not agree with the use of gloves when examining patients with MRSA and 34% did not believe in the efficacy of alcohol hand rub solutions in reducing MRSA transmission, concluding that "there is scope for improvement in awareness and knowledge about MRSA and its management". Acknowledging this problem, the recent CDC guidelines40 advise that "improvement requires that the organizational leadership make prevention an institutional priority and integrate infection control practices into the organization's safety culture".
The high endemic values detected in our study as well as differences in the perception of the HCP's about the epidemiology and management of MRSA positive patients justify the need to implement a global infection control programme for MRSA, with consensus-based measures for management of colonized and infected patients, more effective strategies for the rational use of antimicrobials, as well as more dynamic and focused educational programmes for healthcare professionals.
References
1. European Antimicrobial Resistance Surveillance System. European Antimicrobial Resistance Surveillance System Annual Report 2005. [Internet]. Bilthoven: EARSS; 2005. [cited 2008 April 27]. Available from: www.rivm.nl/bibliotheek/rapporten/210624001.pdf. [ Links ]
2. Grundmann H, Aires–De–Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin–resistant Staphylococcus aureus as a public–health threat. Lancet. 2006;368:874–85. [ Links ]
3. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin–susceptible and methicillin–resistant Staphylococcus aureus. Arch Intern Med. 2002;62:2229–35. [ Links ]
4. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin–resistant and methicillin–susceptible Staphylococcus aureus bacteremia: a meta–analysis. Clin Infect Dis. 2003;36:53–9. [ Links ]
5. Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36:592–8. [ Links ]
6. Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin–resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. [ Links ]
7. Kim T, Oh PI, Simor AE. The economic impact of methicillin–resistant Staphylococcus aureus in Canadian hospitals. Infect Control Hosp Epidemiol. 2001;22:99–104. [ Links ]
8. Appelbaum C. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin Infect Dis. 2007;45 Suppl 3:S165–70. [ Links ]
9. Kluytmans–Vandenbergh MF, Kluytmans JA. Community–acquired methicillin–resistant Staphylococcus aureus: current perspectives. Clin Microbiol Infect. 2006;12 Suppl 1:9–15. [ Links ]
10. Chaberny IF, Sohr D, Rüden H, Gastmeier P. Development of a surveillance system for methicillin–resistant Staphylococcus aureus in German hospitals. Infect Control Hosp Epidemiol. 2007;28:446–52. [ Links ]
11. Henderson DK. Managing methicillin–resistant Staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms. Am J Med. 2006;119(6 Suppl 1):S45–52. [ Links ]
12. Garner J, Hospital Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention, Public Health Service, U. S. Department of Health and Human Services. Guideline for isolation precautions in hospitals: Part II: recommendations for isolation precautions in hospitals. Am J Infect Control.1996;24: 32–52. [ Links ]
13. Health Ministry, National Infection Control Programme. Epidemiological surveillance protocol for bloodstream nosocomial infections. Lisbon: National Health Institute Dr. Ricardo Jorge. National Infection Control Programme; 2004. [ Links ]
14. Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al. Sistematic review of isolation policies in the hospital management of the methicillin–resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling. Health Technol Assess. 2003;7:1–194. [ Links ]
15. Ducel G, Fabry J, Nicolle L. Prevention of hospital acquired infections: a practical guide. 2nd ed. Copenhagen: World Health Organization; 2002. [ Links ]
16. Horan T, Gaynes R: Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital epidemiology and infection control. 3rd ed. Philadelphia, PA: Lippincott Willians & Wilkins; 2004. p. 1659–1702. [ Links ]
17. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: fifteenth informational supplement. 15th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2005. [ Links ]
18. Viera AJ, Garrett JM. Understanding interobserver agreement: the Kappa Statistic. Fam Med. 2005;37:360–3. [ Links ]
19. Madani TA, AL–Abdullah NA, AL–Sanousi AA, Ghabrah TM, Afandi SZ, Bajunid HA. Methicillin–resistant Staphylococcus aureus in two tertiary–care centers in Jeddah, Saudi Arabia. Infect Control Hosp Epidemiol. 2001;22:211–6. [ Links ]
20. Montesinos I, Salido E, Delgado T, Lecuona M, Sierra A. Epidemiology of methicillin–resistant Staphylococcus aureus at a university hospital in the Canary Islands. Infect Control Hosp Epidemiol. 2003;24:667–72. [ Links ]
21. Fluit AC, Wielders CL, Verhoef J, Schmitz FJ. Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study. J Clin Microbiol. 2001;39:3727–32. [ Links ]
22. Chaves F, Garcia–Martinez J, De Miguel S, Sanz F, Otero JR. Epidemiology and clonality of methicillin–resistant and methicillin–susceptible Staphylococcus aureus causing bacteremia in a tertiary–care hospital in Spain. Infect Control Hosp Epidemiol. 2005;26:150–6. [ Links ]
23. Monnet DL, Mackenzie FM, Lopez–Lozano JM, Beyaert A, Camacho M, Wilson R, et al. Antimicrobial drug use and methicillin–resistant Staphylococcus aureus, Aberdeen, 1996–2000. Emerg Infect Dis. 2004;10:1432–41. [ Links ]
24. Marschall J, Mühlemann K. Duration of methicillin–resistant Staphylococcus aureus carriage, according to risk factors for acquisition. Infect Control Hosp Epidemiol. 2006;27:1206–12. [ Links ]
25. Weber SG, Gold HS, Hooper DC, Karchmer AW, Carmeli Y. Fluoroquinolones and the risk for methicillin–resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003;9:1415–22. [ Links ]
26. Fishbain JT, Lee JC, Nguyen HD, Mikita JA, Mikita CP, Uyehara CF, et al. Nosocomial transmission of methicillin–resistant Staphylococcus aureus: a blinded study to establish baseline acquisition rates. Infect Control Hosp Epidemiol. 2003;24:415–21. [ Links ]
27. Sa–Leao R, Sanches IS, Couto I, Alves CR, De Lencastre H. Low prevalence of methicillin–resistant strains among Staphylococcus aureus colonizing young and healthy members of the community in Portugal. Microb Drug Resist. 2001;7:237–45. [ Links ]
28. Melo–Cristino J, Alves AF, Amorim JM, Diogo J, Lito LM, Lopes P, et al. Estudo multicêntrico de resistência aos antimicrobianos em nove hospitais portugueses: comparação de resultados num intervalo de uma década. Revista Portuguesa de Doenças Infecciosas. 2006;3:7–15. [ Links ]
29. Meyer E, Schwab F, Gastmeier P, Jonas D, Rueden H, Daschner FD. Methicillin–resistant Staphylococcus aureus in German intensive care units during 2000–2003: data from Project SARI (Surveillance of Antimicrobial Use and Antimicrobial Resistance in Intensive Care Units). Infect Control Hosp Epidemiol. 2006;27:146–54. [ Links ]
30. Albertini MT, Benoit C, Berardi L, Berrouane Y, Boisivon A, Cahen P, et al. Surveillance of methicillin–resistant Staphylococcus aureus (MRSA) and Enterobacteriaceae producing extended–spectrum beta–lactamase (ESBLE) in Northern Fran a five–year multicentre incidence study. J Hosp Infect. 2002;52:107–13. [ Links ]
31. Richet HM, Benbachir M, Brown DE, Giamarellou H, Gould I, Gubina M, et al. Are there regional variations in the diagnosis, surveillance, and control of methicillin–resistant Staphylococcus aureus? Infect Control Hosp Epidemiol. 2003; 24:334–41. [ Links ]
32. Klevens RM, Morrison MA, Fridkin SK, Reingold A, Petit S, Gershman K, et al. Community–associated methicillin–resistant Staphylococcus aureus and healthcare risk factors. Emerg Infect Dis. 2006;12:1991–3. [ Links ]
33. Salgado CD, Farr BM. What proportion of hospital patients colonized with methicillin–resistant Staphylococcus aureus are identified by clinical microbiological cultures? Infect Control Hosp Epidemiol. 2006;27:116–21. [ Links ]
34. Berhe M, Edmond MB, Bearman, GM. Practices and an assessment of health care workers'' perceptions of compliance with infection control knowledge of nosocomial infections. Am J Infect Control. 2005;33:55–7. [ Links ]
35. Boyce JM, Pittet D. Guideline for hand hygiene in health care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/ SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR. 2002;51 (RR–16):1–44. [ Links ]
36. Solberg CO. Spread of Staphylococcus aureus in hospitals: causes and prevention. Scand J Infect Dis. 2000;32:587–95. [ Links ]
37. Shiomori T, Miyamoto H, Makishima K, Yoshida M, Fujiyoshi T, Udaka T, et al. Evaluation of bedmaking–related airborne and surface methicillin–resistant Staphylococcus aureus contamination. J Hosp Infect. 2002;50:30–5. [ Links ]
38. Afif W, Huor P, Brassard P, Loo Vg. Compliance with methicillin–resistant Staphylococcus aureus precautions in a teaching hospital. Am J Infect Control. 2002;30:430–3. [ Links ]
39. Seaton RA, Montazeri AH. Medical staff knowledge about MRSA colonization and infection in acute hospitals. J Hosp Infect. 2006;64:297–9. [ Links ]
40. Siegel JD, Rhinehart E, Jackson M, Chiarello L, The Healthcare Infection Control Practices Advisory Committee. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Am J Infect Control. 2007;35(10 Suppl 2):S65–164. [ Links ]
Acknowledgments
Financial support: "Programa Operacional Ciência e Inovação 2010", supported by European Social Funding and Portuguese Ministry of Science and Technology (Fundação para a Ciência, Tecnologia e Ensino Superior).
Received 5 November 2010
Accepted 12 May 2011













