Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias v.32 n.2 Lisboa dez. 2009
Fungi associated to Platypus cylindrus Fab. (Coleoptera: Platypodidae) in cork oak
Fungos associados ao insecto Platypus cylindrus Fab. (Coleoptera: Platypodidae) em sobreiro
Joana Henriques, Maria de Lurdes Inácio, Edmundo Sousa *
ABSTRACT
Platypus cylindrus is a pest that since the 80s of the last century has been considered a cork oak mortality agent in Portugal. It is an ambrosia beetle that establishes complex symbioses with fungi whose role in the insect-fungus-host interaction has not been completely clarified. In order to characterize P. cylindrus associated micoflora in Portugal, fungi were isolated from different beetle organs and from its galleries in cork oak trees. Fungi of the genera Acremonium, Aspergillus, Beauveria, Botrytis, Chaetomium, Fusarium, Geotrichum, Gliocladium, Nodulisporium, Paecilomyces, Penicillium, Raffaelea, Scytalidium, Trichoderma and of the order Mucorales were identified. An actinomycete of the genus Streptomyces was also identified. Some of these genera were related for the first time to this interaction. In the present work the isolated fungi are characterized and their contribution for beetle population establishment and tree weakness is discussed.
Key-words: ambrosia beetle, decline, interaction, mycoflora, Quercus suber.
RESUMO
Platypus cylindrus é uma praga que desde os anos 80 do século passado tem sido referida como agente de mortalidade do sobreiro em Portugal. É um insecto ambrósia que estabelece simbioses complexas com fungos cujo papel não está completamente esclarecido na interacção insecto-fungo-sobreiro. Com o objectivo de caracterizar a micoflora associada a P. cylindrus em Portugal foram efectuados isolamentos a partir de diferentes órgãos do insecto e suas galerias em sobreiro. Identificaram-se fungos dos géneros Acremonium, Aspergillus, Beauveria, Botrytis, Chaetomium, Fusarium, Geotrichum, Gliocladium, Nodulisporium, Paecilomyces, Penicillium, Raffaelea, Scytalidium, Trichoderma e da ordem Mucorales. Foi igualmente identificado um actinomiceta do género Streptomyces. Alguns destes géneros são referidos pela primeira vez nesta interacção. No presente trabalho caracterizam-se os fungos isolados e discute-se a sua contribuição para o estabelecimento das populações do insecto e enfraquecimento das árvores.
Palavras-chave: insecto ambrósia, declínio, interacção, micoflora, Quercus suber.
INTRODUÇÃO
Scolytidae and Platypodidae are among the most successful wood-inhabiting beetles causing damage of economic significance to trees and timber (Cassier et al., 1996). Platypus cylindrus Fab. is a cork oak (Quercus suber L.) pest that has come to assume an increasing importance in Portugal and Mediterranean basin countries (Ferreira & Ferreira, 1989; Chakali et al., 2002; Riziero et al., 2002; Sousa et al., 2005). Until recently, damages produced by this insect were limited to dead or weakened trees. The understanding of recent population outbreaks, mainly in Portuguese cork oak stands, can be based on three assumptions: (i) gradual changes of the cork oak stand dynamics, (ii) development of more specific host colonization mechanisms and (iii) changes on the insects and their natural enemies population dynamics (Sousa & Inácio, 2005). Within the host colonization strategies, its essential to consider the fungal symbiosis that may contribute to the host weakness and create better conditions for the establishment of insects (Henriques et al., 2006).
In deed, P. cylindrus, as almost all members of the Platypodidae, is denominated an ambrosia beetle because larvae and adults feed mainly on fungi (ambrosia fungi) that cover the gallery walls (Batra, 1963). This insect-fungi relation is expressed in an ectosymbiosis in which the fungi live outside the insects body but are temporarily stored in special ectodermical organs for dissemination purposes (Francke-Grosmann, 1967). Insects carry viable inoculum in sac-like structures, called mycangia, located in the prothorax; the inoculum is protected from desiccation during the entire life of the beetle and is disseminated into new breeding sites at the time of tunnel excavation (Batra, 1963; Sousa & Inácio, 2005).
Ambrosia fungi definition includes a set of concepts whose interception allows the classification of several fungi as ambrosia: i) direct participation in insect feeding; ii) presence inside insect galleries in the host; iii) dimorphism, meaning ability to grow both as yeast and mycelia; iv) possible specificity in the insect-fungi-host relationship (Batra, 1963; Beaver, 1989).
Batra (1985) grouped ambrosia fungi as primary and auxiliary. Primary ambrosia fungi are highly insect species specific and their distribution correspond to those of insect symbionts. They are present and dominant in the tunnels and isolated regularly from the mycangia of the beetles in the flight stage or when excavating tunnels. Auxiliary ambrosia fungi are transitory, non-specific with respect to symbiont insect and may appear after insect development. They may not be present in larval cradles or in adult beetles; and their habitat and distribution range are unrestricted and unrelated to that of the ambrosia beetles.
Several fungi have already been isolated from P. cylindrus and from galleries in Quercus spp. (Baker, 1963; Cassier et al., 1996; Sousa et al., 1997; Morelet, 1998; Henriques et al., 2006). Their constant presence associated to the insect allows us to presume that they play an important role in the symbiosis. Besides the implication in insect feeding, ambrosia fungi might also be involved in other processes such as host weakness, through pathogenic action; decomposition of lignocellulolitic compounds, which helps gallery construction and enables fungi colonization; and/or antagonism that controls the growth of other fungi (Sousa & Inácio, 2005; Henriques et al., 2006).
The aim of the present work is to characterize the micoflora associated to P. cylindrus and discuss its contribution for beetle populations establishment and host weakness.
MATERIAL AND METHODS
Four infested logs of cork oak trees exhibiting decline symptoms were collected from three of the main Portuguese cork oak productive regions: Chamusca (Ribatejo province), Montemor and Grândola (Alentejo province). The logs were maintained in the laboratory in order to capture 100 P. cylindrus insects, males and females, as they emerged. Samplings were repeated during 2005, 2006 and 2007. The insects were aseptically dissected in mycangia, intestine and parts of the exoskeleton (elytra). The logs were cut in order to identify the different insect gallery sections: cork, inner-bark, pre-parental section, larval section and gallery end. One complete gallery was observed from each log and six samples (fragments with 1 cm2) of each section were collected.
The samples were surface sterilized with a sodium hypochlorite solution (1%) for 1 min, rinsed with distilled sterilized water and then plated in malt extract agar (MEA, Difco, USA) added with streptomycin (500 mg/l) and MEA added with cycloheximide (500 mg/l). The cultures were incubated at 24±1ºC in darkness. Pure cultures of each fungi isolate were obtained and identified as genus based on morphological features according to Ellis (1971, 1976), Lanier et al. (1978), Kiffer & Morelet (1997) and Barnett & Hunter (1998). Cultural description of colonies was made on potato dextrose agar (PDA, Difco, USA).
RESULTS
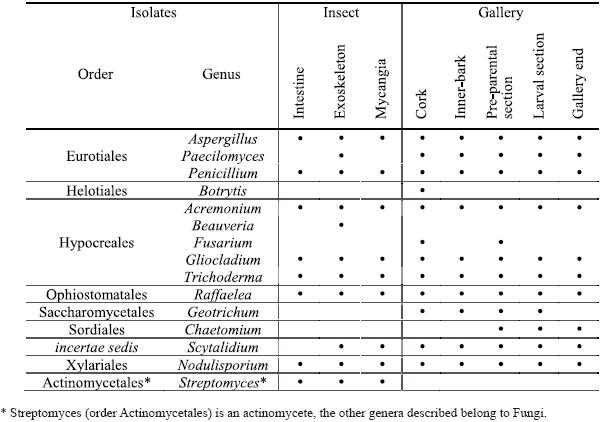
Fifteen genera were isolated from the different insect parts and from all cork oak gallery sections (Table 1). The identified fungi are classified in very distinct orders including the Ascomycota (Eurotiales, Heliotioales, Hypocreales, Ophiostomatales, Saccharomycetales, Sordiales and a genus not assigned to any order) and Basidiomycota (Xylariales) although all the obtained genera, except Chaetomium, were isolated in the mitosporic state. Also, an actinomycete was isolated from the insect (Streptomycetales).
Table 1 – Genera isolated from Platypus cylindrus intestine, exoskeleton and mycangia and from the different sections of insects galleries on cork oak.
The isolated genera were described according to the morphological features based on optical microscopic observations and literature guides. In table 2 the main cultural features for all isolated genera are described.
Table 2 – Cultural characteristics on PDA of the isolated genera.
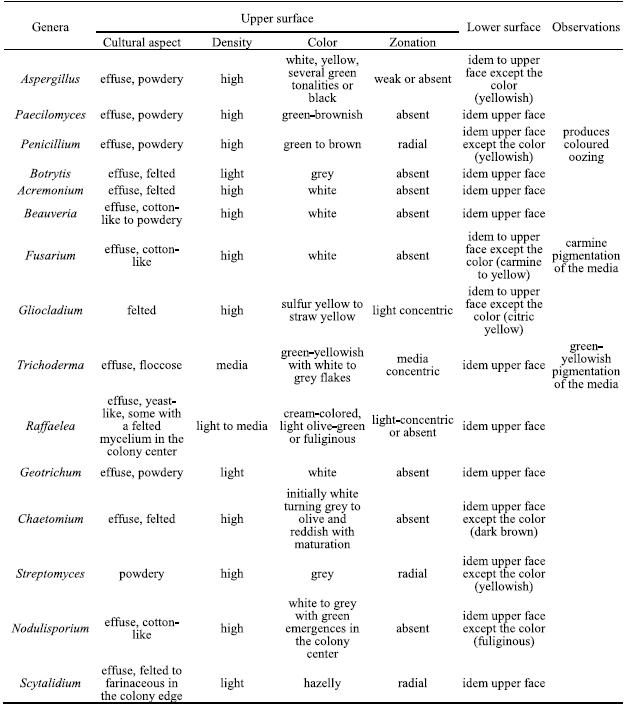
From the genus Aspergillus several individuals with different cultural characteristics were isolated, probably belonging to different species. Their microscopic features are: hyphae septate and branched, conidiophores macronematous, mononematous, erect, simple, often with a foot cell, with a terminal vesicle bearing short branches or phialides radiating from the entire surface. When present, the branches are in one or several series and the terminal ones always bear phialides. Conidiogenic cells monophialidic, discrete, several arising together at the end of terminal branches or over the surface of the vesicles, mostly determinate, rarely percurrent, ampulliform or lageniform, collarettes sometimes present. Conidia are phialosporic, unicellular, dry, smooth, rugose, echinulate, globose and hyaline but colored in mass, disposed in long basiptal chains (Figure 1a).
Penicillium produces conidiophores macronematous, mononematous, simple or branched, penicilated, ending in a group of phialides. The walls of conidiophores may be smooth and thin or variously roughened, with aerial portions appearing delicately echinulate, granular or asperulate. The penicillus covers all the branching system and can be monoverticillate or biverticillate, symmetrical or asymmetrical. Conidia are phialosporic, unicellular, dry, globose or ovoid, hyaline to green (Figure 1b).
Paecilomyces produces conidiophores macronematous, mononematous, arising as upright branches from hyphal ropes or aerial mycelium, smooth-walled, usually with several stages of irregular branching, and frequently bearing secondary branches, with divergent penicilated phialides, cylindrical to ellipsoidal in the lower part, usually narrowing abruptly into a long cylindrical neck. Conidia phialosporic, unicellular, hyaline, ovoid, smooth-walled, highly variable in size produced in long, strongly divergent chains (Figure 1c).
Several Raffaelea isolates were obtained showing a great macro and microscopic variability, probably corresponding to different species. This genus produces hyphae hyaline and septate that bound together forming compact hyphae ropes. Superficial sporodochia, effuse, white to brownish. Conidiophores are macronematous and mononematous, erect, septate, slender with a tapered apex, producing simpodulosporic conidiae that leave cicatricial scars in the conidiogenous cells. Conidiae are unicellular and hyaline, with variable forms (triangular, oval, allantoid, fusiform or truncated claviform) and dimensions (Figure 1d).
Fusarium produces conidiophores macronematous and mononematous, variable, slender, and simple or stout, short, septate and branched that originates phialosporic conidia, hyaline, canoe-shaped, with 5 to 6 transversal septa and collected in a slimy drop. No microconidia were observed (Figure 1e).
Trichoderma conidiophores macronematous and mononematous, hyaline and highly ramified, disposed in pyramidal structures with inserted phialides in 90º angles. Conidia are phialosporic, enteroblastic and monoblastic, unicellular, green, globose to subglobose and remain grouped in the top of the phialides (Figure 1f).
Beauveria produces conidiophores micronematous and mononematous, simple, irregularly grouped, inflated in the base and tapered in the apical fertile portion that appears sinusoidal after conidia production. Conidia are simpodulosporic, unicellular, dry, hyalines and ovoid with small denticles, giving the conidiogenous cells a spiny appearance (Figure 1g).
Gliocladium presents conidiophores macronematous and mononematous, erect, septate and branched, ending in a branched system of phialides disposed in tight penicilate structure with three phialides per metulae. Phialides narrowly cylindrical to sub-ulate, taper slightly towards the tip, smooth-walled. Conidia are phialosporic, unicellular, hyaline, ovoid or cylindrical and aggregated in conidial masses, slimy to watery, whitish or light-yellow, never forming imbricate chains (Figure 1i).
Acremonium produces hyphae hyaline septate that sometimes bound together by anastomoses. Conidiophores are macronematous and mononematous, erect, solitary to weakly branched, straight, tapered to the apex, with basal septum to separate the conidiophore from the vegetative hyphae. Conidia are phialosporic, unicellular and ovoid, aggregated in a slimy drop (Figure 1k).
Geotrichum produces conidiophores micronematous and mononematous producing arthrosporic conidiae, hyaline, unicellular, dry, smooth, short cylindrical with truncated bases, resulting from fragmentation of undifferentiated hyphae by fission through double septum, (Figure 1l).
Botrytis has conidiophores macronematous, mononematous, straight or flexuous, smooth, brown, slender, irregularly branched with enlarged apical polyblastic cells where botryoblatosporic conidia are produced simultaneously; conidia solitary, simple, smooth, hyaline to grey in mass, unicellular, ovoid with a denticule. Mycelium immersed or superficial, with brownish course hyphae. Sclerotia frequently present (Figure 1h).
Chaetomium produces superficial perithecia, brown, single, globose, covered with different sized brown filaments; asci clavate, pedunculate with evanescent walls. Ascospores unicellular, light olive-brown and lemoniform, smooth, often pushed out of ostiole in a cirrhus (Figure 1j).
Streptomyces produces filaments that originate very small spores by fragmentation that remain disposed in helical chains (Figure 1m).
Nodulisporium produces mycelium partly immersed and partly superficial, conidiophores macronematous and mononematous, arising laterally from the brownish vegetative hyphae, with principal axis erect, septate, branched, hyaline to light brown, slightly rugouse, conidiogenic cells poliblastic and sympodial, slender or short and thick, verticilated. Conidiae sympodulosporic, acropleurogenous, unicellular, hyaline or brown to olive in mass, ellipsoidal or obovoid, smooth or roughened, with a small frill when detached (Figure 1n).
Scytalidium produces sparse thick hyphae, septate, hardly branched, hyaline or light-brown, disposed in parallel; conidiophores micronematous and mononematous with terminal conidiogenic cell originating arthroconidiae by holothalic fragmentation. Conidia are catenulated, schizogenous, uni or bicellular, hyaline, rectangular. It also forms terminal or interpolate chlamydospores, with thick wall, brown and ellipsoidal (Figure 1o).
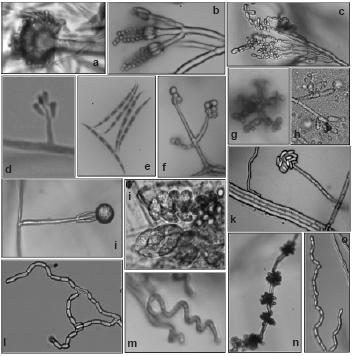
Figure 1 – Reproductive structures of isolated genus: a) Aspergillus (x1000); b) Penicillium (x1000);c) Paecilomyces (x600); d) Raffaelea (x600); e) Fusarium (x600); f) Trichoderma (x600); g) Beauveria (x1000);h) Botrytis (x600); i) Gliocladium (x400); j) Chaetomium (x1000); k) Acremonium (x600); l) Geotrichum; m) Streptomyces (x1000); n) Nodulisporium (x600); o) Scytalidium (x600).
In P. cylindrus galleries on cork oak, greater fungi variety were presented than in the insects body. In both cases were isolated cosmopolite fungi and others more specific for insect-fungi relation. Concerning isolates from insects, it was in the exoskeleton that was found the major diversity of genus, followed by mycangia and then intestine. Among sexes, no relevant differences were observed. Penicillium, Raffaelea and Aspergillus were the most frequent genus in the three sampled organs and for both sexes. Beauveria and Streptomyces were only isolated from the insect, the former only from the exoskeleton.
Along P. cylindrus galleries in cork oak, the majority of the isolated genera were found in all sections and, in general, in similar proportions, highlighting cosmopolite and saprophytic fungi such as Penicillium, Trichoderma, Gliocladium and Scytalidium, but also Raffaelea was isolated in a considerable percentage in all sections. Botrytis, Fusarium, Geotrichum and Chaetomium were isolated only from the galleries but in very low percentages and distribution.
In Figures 2 and 3 are represented the distribution of the isolated fungi both in P. cylindrus body and along its galleries in cork oak, respectively.
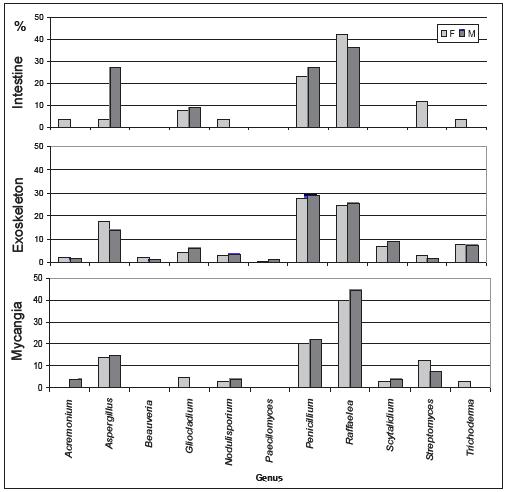
Figure 2 – Percentage of isolated genera in the different body parts (intestine, exoskeleton and mycangia) of Platypus cylindrus females (F) and males (M).
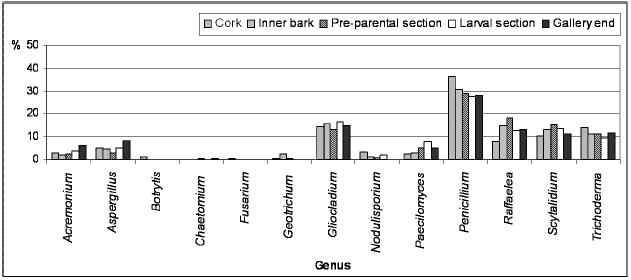
Figure 3 – Percentage of isolated genera from the different Platypus cylindrus galleries on cork oak (cork, inner bark, pre-parental section, larval section and gallery end).
DISCUSSION
From all isolated genera, Raffaelea is highlighted because of its known importance as ambrosia fungi and both its frequency and distribution (Beaver, 1989). This genus includes twelve species, the majority associated with ambrosia beetles (Kubono & Ito, 2002; Bisby et al., 2006). Two species (R. ambrosiae and R. montetyi) are identified as P. cylindrus primary ambrosia fungi (Arx & Hennebert, 1965; Morelet, 1998) and have been isolated both from the insect organs and from its galleries in the host (Sousa et al., 1997; Morelet, 1998). Although Raffaelea sexual state is lacking, observations of conidial development support its placement within the Ophiostomatales (Gebhardt & Oberwinkler, 2005) and, 18S ribosomal DNA sequence analysis, shows a monophyletic lineage which forms a sister group to species of the genus Ophiostoma (Jones & Blackwell, 1998). The Ophiostomatales are economically important sapstaining fungi occurring world-wide on hardwoods and commercially produced pines, and are in some cases, already known as pathogenic to oaks (Degreef, 1992). The effect of Raffaelea spp. on cork oak remains unknown, but in Japan, R. quercivora involved in an interaction with P. quercivorus has proven pathogenicity to several oak trees (Kubono & Ito, 2002; Kinnura & Kobayashi, 2006).
Other isolated fungi were already related to P. cylindrus. Sousa et al. (1997) isolated A. carbonarius from mycangia and cork oak galleries. Pa. variotii has been isolated from galleries in cork oak and other oaks (Baker, 1963). Also Penicillium was found both on the insect and in oak galleries (Baker, 1963; Cassier et al., 1996; Sousa et al., 1997). Botrytis was isolated from galleries in oak, as well as Acremonium and Fusarium (Baker, 1963). Acremonium sp. and F. solani, Gliocladium roseum and G. solani has also been isolated by Sousa et al. (1997) from P. cylindrus organs and galleries in cork oak. Cassier et al. (1996) isolated Trichoderma from the insect and T. viride was isolated from galleries on cork oak and other oaks (Baker, 1963; Sousa et al., 1997). Nodulisporium and Scytalidium were found in mycangia and galleries on cork oak, the last one only in the host (Sousa et al., 1997).
Four genera were newly associated with this interaction: Beauveria, Geotrichum, Chaetomium and Streptomyces. Beauveria is an entomopathogenic fungus and two species (B. bassiana and B. brongniartii) are already known as harmful to Platypus spp. (Glare et al., 2002). Several species of Geotrichum have been identified in association with insects (Suh & Blackwell, 2006) and the genus Streptomyces act as an antimicrobial defense in the termite-fungi association (Mueller & Gerardo, 2002). Chaetomium is a very common genus in soil and plant debris, in particular in wood (Hawksworth, 1995) and it might be a Scytalidium teleomorph (Halin, 1997).
The role of each isolated genus in the in-sect-fungi-host interaction is discussed. Most of them are considered cosmopolite and saprophytic fungi (Hawksworth et al., 1995; Kiffer & Morelet, 1997) which can be involved just in a commensalistic relation or might play some other action due to their isolation frequency and distribution among samples.
Following P. cylindrus host colonization process, symbiotic fungi may start to act as wood degrading, thus facilitating galleries excavation. Trichoderma, as a great extrolite producer such as lignocellulolictic enzymes, might be an active intervenient in that process (Samuels, 1996). Then, to overcome host defense reaction, the insect might inoculate phytopathogenic fungi. Nodulisporium might be relevant in this part as the ITS rDNA analysis proved that it is a Biscogniauxia mediterranea anamorph, which is responsible for cork oak charcoal disease (Collado et al., 2001). Other isolated genera might also interfere in this phase: Acremonium which might be Nectria anamorph, thus being a potential pathogenic to several woody plants and Fusarium also pathogenic to several plant organs (Kiffer & Morelet, 1997). Within the galleries, fungi that will be nourishment for larvae develop in a controlled manner by the permanent care of parental insects but probably also due to the antagonistic action of fungi over others. Gliocladium and Trichoderma are known by their antagonistic activity being used as biocontrol agents (Papavizas, 1985). Streptomyces and Scytalidium might act in the fungi colonies management within the galleries. Also in the insect body this management might be essential to achieve ambrosia fungi transport. The final role of the isolated fungi, which is the base of this insect-fungi interaction, is the larvae nourishment.
According to definition, ambrosia fungi are eaten by the insects and thus are the ones found in their intestine. Nevertheless, whereas larvae eat exclusively ambrosia fungi, the adults might also eat wood, which explains all the cosmopolite and saprophytic fungi found in their intestine that were already contaminating tree wood. Also, in mycangia, where specific fungi for this interaction are transported, ambrosia fungi are expected to be present. In both cases, intestine and mycangia, Raffaelea was the most frequent genus, which supports the fact that some species of this genus are P. cylindrus primary ambrosia fungi. The other isolated fungi might be considered auxiliary ambrosia fungi as sustained by Batra (1985) or simply be worldwide saprobes that are frequently present in host tissues.
In conclusion, there is a vast diversity of fungi associated to P. cylindrus-cork oak interaction. Some could be determinant to the success of the insect colonization, mainly Raffaelea which is its principal ambrosia fungi and also might be pathogenic to host trees.
Acknowledgments
The authors wish to thank to Professor Arlindo Lima (Departamento de Protecção de Plantas e Fitoecologia, Instituto Superior de Agronomia) for critical comments on the manuscript.
BIBLIOGRAPHIC REFERENCES
Arx, J.A. von & Hennebert, G.L. (1965) -Deux champignons ambrosia. Mycopathologia et mycologia applicata 25: 309-315.
Baker, J.M. (1963) -Ambrosia beetle and their fungi, with particular reference to Platypus cylindrus Fab. Symposia of the Society for General Microbiology 13: 323-354.
Barnett, H.L. & Hunter, B.B. (1988) - Illustrated Genera of Imperfect Fungi. APS Press, Minnesota, USA, 218 pp.
Batra, L.R. (1963) -Ecology of ambrosia fungi and their dissemination by beetles. Transactions of the Kansas Academy of Science 66: 213-236.
Batra, L.R. (1985) -Ambrosia beetle and their associated fungi: Research trends and techniques. Proceedings of the Indian Academy of Sciences 49: 137-148.
Beaver, R.A. (1989) -Insect-fungus relationships in the bark and ambrosia beetles. In: Wilding, N.; Collins, N.M.; Hammond, P.M. & Webber, J.F. (Eds.) Insect-Fungus Interactions. Academic Press, London, pp. 121-143.
Bisby, F.A.; Ruggiero, M.A.; Roskov, Y.R.;Cachuela-Palacio, M.; Kimani, S.W.; Kirk, P.M.; Soulier-Perkins, A. & van Hertum, J. (2006) -Species 2000 & ITIS Catalogue of Life: 2006 Annual Checklist. Available in <http://www.sp2000.org.> (accessed in: 15 November 2007).
Cassier, P.; Lévieux, J.; Morelet, M. & Rougon, D. (1996) - The mycangia of Platypus cylindrus Fab. and P. oxyurus Dufour (Coleoptera: Platypodidae). Structure and associated fungi. Journal of Insect Physiology 42: 171-179.
Chakali, G.; Attal-Bedreddine, A. & Ouzani, H. (2002) - Insect pests of the oaks Quercus suber and Q. ilex in Algeria. IOBC/ wprs Bulletin 25: 93-100.
Collado J.; Platas, G. & Peláez, F. (2001) -Identification of an endophytic Nodulisporium sp. from Quercus ilex in central Spain as the anamorph of Biscogniauxia mediterranea by rDNA sequence analysis and effect of different ecological factors on distribution of the fungus. Mycologia 93: 875-886.
Degreef, J. (1992) - Isolation of Ophiostoma querci (Georgev.) Nannfeldt from declining oaks in Belgium: selection techniques and pathogenicity test. In: Proceedings of an International Congress Recent Advances in Studies on oak decline. Selva di Fasano (Brindisi), Italy, pp. 471-473.
Ellis, M.B. (1971) - Dematiaceous Hyphomycetes. CAB, England, 608 pp.
Ellis, M.B. (1976) -More Dematiaceous Hyphomycetes. CAB, England, 507 pp.
Ferreira, M.C. & Ferreira, G.W.S. (1989) -Platypus cylindrus F. (Coleoptera: Platipodidae) Plaga de Quercus suber. Boletin de Sanidade Vegetal Plagas 4: 301-305.
Francke-Grosmann, H. (1967) -Ectosymbiosis in wood-inhabiting insects. In: S.M. Henry (Ed.) Symbiosis, its physical and biochemical significance. Academic Press, New York, pp. 141-203.
Gebhardt, H. & Oberwinkler, F. (2005) - Conidial development in selected ambrosial species of the genus Raffaelea. Antoine van Leeuwenhoek 88: 61-66.
Glare, T.R.; Placet, C.; Nelson, T.L. & Reay, S.D. (2002) -Potencial of Beauveria and Metarhizium as control agents of pinhole borers (Platypus spp.). New Zealand Plant Protection 55: 73-79.
Halin, R.T. (1997) - Ilustrated Genera of Ascomycetes. Vol I., APS Press, Minnesota, 263 pp.
Hawksworth, D.L.; Kirk, P.M.; Sutton, B.C. & Pegler, D.N. (1995) -Ainsworth & Bisbys Dictionary of the Fungi. CAB International, UK, 616 pp.
Henriques, J.; Inácio, M.L. & Sousa, E. (2006) - Ambrosia fungi in the insect-fungi symbiosis in relation to cork oak decline. Revista Iberoamericana Micologia 23: 185-188. [ Links ]
Jones, K.G. & Blackwell, M. (1998) - Phylogenetic analysis of ambrosial species in the genus Raffaelea based on 18S rDNA sequences. Mycologycal Research 102: 661-665.
Kiffer, E. & Morelet, M. (1997) -Les Deutèromycetes – classification et clés d´identification générique. INRA Editions, Paris, 306 pp.
Kinuura, H. & Kobayashi, M. (2006) - Death of Quercus crispula by inoculation with adult Platipus quercivorus (Coleoptera: Platypodidae). Applied Entomology and Zoology 41, 1: 123-128.
Kubono, T. & Ito, S. (2002) - Raffaelea quercivora sp. nov. associated with mass mortality of Japanese oak, and the ambrosia beetle (Platypus quercivorus). Mycoscience 43: 255-260.
Lanier, L.; Joly, P.; Bondoux, P. & Bellemère; A. (1978) -Mycologie et pathologie forestières. Tome I - Mycologie forestière. Masson, Paris. 487 pp.
Morelet, M. (1998) - Une espèce nouvelle de Raffaelea, isolée de Platypus cylindrus, coléoptère xylomycétophage des chênes. Extrait des Annales de la Société des Science Naturelles et dArchéologie de Toulon et du Var 50: 185-193.
Mueller, U.G. & Gerardo, N. (2002) - Fungus-farming insects: Multiple origins and diverse evolutionary histories. Proceedings of the National Academy of Sciences 99, 24: 15247-15249.
Papavizas, G.C. (1985) -Trichoderma and Gliocladium: biology, ecology and potential for biocontrol. Annual Review Phytopathology 23: 23-54.
Riziero, T.; Ragazzi A.; Marianelli, L.; Sabbatini, P. & Roversi, P.F. (2002) - Insects and fungi involved in oak decline in Italy, IOBC/wprs Bulletin 25, 5: 67-74.
Samuels, G.J. (1996) - Trichoderma: a review of biology and systematics of the genus. Mycological Research 100, 8: 923-935.
Sousa, E. & Inácio, M.L. (2005) - New Aspects of Platypus cylindrus Fab. (Coleoptera: Platypodidae) Life History on Cork Oak Stands in Portugal. In: F. Lieutier & D. Ghaioule (Eds.) Entomological Research in Mediterranean Forest Ecosystems. INRA Editions, Paris, 280 pp
Sousa, E.; Inácio, M.L.; El Antry, S.; Bakry, M. & Kadiri, Z.A. (2005) -Comparaison de la bio-écologie et du comportement de linsecte Platypus cylindrus Faber. (Coléoptère, Platypodidae) dans les subéraies Portugaises et Marocaines. IOBC/wprs Bulletin 28: 137-144.
Sousa, E., Tomaz, I. L.; Moniz, F.A. & Basto, S. (1997) -La répartition spatia-le dês champignons associés à Platypus cylindrus Fab. (Coleoptera: Platypodidae). Phytopathologia Medierranea. 36: 145:153.
Suh, S.O. & Blackwell, M. (2006) -Three new asexual asthroconidial yeasts, Geotrichum carabidarum sp. nov., Geotrichum histeridarum sp. nov., and Geotrichum cucujoidarum sp. nov., isolated from the gut of insects. Mycological Research 110: 220-228.
*Instituto Nacional de Recursos Biológicos, I.P. Edifício da ex-Estação Florestal Nacional, Quinta do Marquês, 2780-159 Oeiras joana.henriques@efn.com.pt; lurdes.inacio@efn.com.pt; edmundo.sousa@efn.com.pt
Recepção/Reception: 2009.01.15
Aceitação/Acception: 2009.05.14














