Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias v.34 n.1 Lisboa jan./jun. 2011
Extractability of aluminium and forms in solution of soils derived from granite in Portugal
Edgardo Auxtero1 and Manuel Madeira1
1 Centro de Estudos Florestais, Instituto Superior de Agronomia, Universidade Técnica de Lisboa, Tapada da Ajuda, 1399-017 Lisboa, Portugal (eauxtero@iol.pt)
ABSTRACT
Extractable aluminium (Al) was determined in surface and subsurface horizons of eleven pedons derived from granite occurring in Portugal, under a wide range of precipitation. Five successive extractions with 1 M KCl, 0.5 M CuCl2 and 0.33 M LaCl3 were used. Monomeric (Alm) and organic (Alorg) forms of Al in the soil solution, extracted at field capacity, were also determined. Amounts of extracted Al were greatest with the 0.5 M CuCl2 and lowest with the 1 M KCl. These amounts showed strong positive correlation with soil organic C contents. Soil horizons having high contents (56-80 g kg-1) of organic C showed the highest amount of extractable Al by the 0.5 M CuCl2 (12.2-30.9 cmolc kg-1). Organically bound Al extracted by the 0.5 M CuCl2 and 0.33 M LaCl3 was also highest in these soils (7.6-10.1 and 4.7-8.3 cmolc kg-1, respectively). In contrast, lowest amounts of Al extracted by the 1 M KCl, 0.5 M CuCl2 and 0.33 M LaCl3 (0.9-4.3, 4.1-17.1 and 2.2-7.2 cmolc kg-1, respectively) were observed in horizons containing low organic C content (3-22 g kg-1). Concentrations of total (AlT), monomeric (Alm) and organic (Alorg) Al in the soil solution of studied soils also increased with increasing soil organic C content, but only Alm was significantly correlated with soil organic Ccontent. Solution of soils having low organic C contents (3-6 g kg-1) showed the lowest concentrations of AlT, Alm and Alorg (0.09-1.60, 0.06-0.38 and 0.03-1.29 µg mL-1, respectively). In contrast, the concentrations of AlT (7.1-17.2 µg mL-1),Alm (0.8-0.9 µg mL-1),and Alorg (7.6-16.9 µg mL-1)were much higher in soils containing high amounts of organic C (37-73 g kg-1). In order to monitor the effects of Al in the soil and quality of sub-superficial water, changes in the concentration of monomeric forms of Al and their activities in the soil solution need further study.
Keywords: Aluminium, organic carbon, pH, soil solution.
Alumínio extraível e formas de AL na solução de solos derivados de rocha granítica em Portugal
RESUMO
O alumínio (Al) extraível foi determinado em onze pedones (horizontes surperficiais e subsuperficiais) desenvolvidos sobre formações graníticas, em Portugal, sob condições de precipitação média anual bastante diferenciadas. Para o efeito efectuaram-se cinco extracções sucessivas com KCl 1 M, CuCl2 0.5 M e LaCl3 0.33 M. Foram ainda determinadas as formas monoméricas (inorgânicas) e orgânicas de Al na solução do solo extraídas nas condições de capacidade de campo dos mesmos horizontes. Os teores mais elevados de Al extraídos foram obtidos por CuCl2 0.5 M e os mais baixos por KCl 1 M; em qualquer dos casos observou-se uma forte correlação positiva com o teor de C orgânico no solo. Os teores de Al extraído por KCl 1 M, LaCl3 0.33 MeCuCl2 0.5 M(5.4-7.4, 8.4-10.1, 18.0-28.9 cmolc kg-1, respectivamente)foram mais elevados em solos com elevado teor de C orgânico (63-80 g kg-1) do que em solos com baixo teor de C orgânico (3-22 g kg-1), em que se observaram teores de 0.9-4.3, 4.1-17.1 e 2.2-7.2 cmolc kg-1 para a extracção com KCl 1 M, CuCl2 0.5 Me LaCl3 0.33 M, respectivamente. As concentrações de Al total (AlT), Al monomérico (Alm) e Al orgânico (Alorg) na solução do solo também apresentaram uma forte variação com o teor de C orgânico, mas só o Alm apresentou correlação significativa com esse teor. Solos com baixo teor de C orgânico mostraram as concentrações de AlT, Alm e Alorg mais baixas (0.09-1.60, 0.06-0.38 and 0.03-1.29 µg mL-1, respectivamente) na solução de solo. Por outro lado, as concentrações foram muito mais elevadas (7.1-17.7, 0.8-0.9 e 7.6-16.9 µg mL-1, respectivamente para o AlT,Alm e Alorg) em solos com elevado teor de C orgânico (3.7-7.3 g kg-1). Os efeitos do Al no solo e na qualidade das águas sub-superficiais carecem de estudos respeitantes às alterações da concentração de Al monomérico e à sua actividade na solução do mesmo.
Palavras-chave: Alumínio, matéria orgânica, pH, solução do solo.
INTRODUCTION
Soils derived from granite in Portugal constitute about 35 % of the territory and are mostly found in the north-west and central part of the country (Madeira and Furtado, 1984; Pereira and Fitzpatrick, 1995). They occur in a wide range of mean annual precipitation (500-3000 mm) and temperature (10-16 ºC). Therefore they show a quite variable profile development and organic matter content (Martins et al., 1995), and could be classified as Regosols, Cambisols, Umbrisols and Leptosols (WRB, 2006). Also, in wetter areas (above 1200 mm), the deeper horizons (poor in organic matter) can contain up to 80 % of gibbsite in the clay fraction, while the superficial horizons show negligible amounts of gibbsite, high organic C content (30-90 g kg-1) and increased amounts of organo-mineral complexes (Madeira and Furtado, 1987; Furtado et al., 1988; Martins et al., 1995). Given the high annual rainfall, it is probable that release of Al from gibbsite-rich horizons may favour the production of various ionic Al species (Lindsay and Walthall, 1989):
Active Al in the soil may be chemically bound to negatively charged clay surfaces by electrostatic forces thus, may also be freely exchangeable with calcium, magnesium or potassium. Aluminium may be present as organic complexes, and as complex hydroxyl polymeric compounds occupying the interstitial spaces of 2:1 clay minerals thus, only partially exchangeable or totally non-exchangeable to other cations (Barnhisel and Bertsch, 1982). Other forms of Al may be present as complexes with phosphate, sulphate and fluoride ions (Fripiat and Herbillon, 1971). Hydrolysis of Al, promotes strongly acid reaction (pH 3.9-4.9) of the local environment, and under this condition trivalent monomeric Al complex is highly dissolved which may be toxic to plants.
In order to minimize the amounts of toxic trivalent Al species in acid soils and natural waters, Al-organic matter interactions in soils, distribution of naturally occurring organic ligands that bind with Al and the stability of Al-organic matter complexes need to be understood for successful management of soils which are dominated by aluminous materials. Some studies (Stevenson and Vance, 1989) suggest that Al3+ toxicity in acid soils may be reduced or eliminated by practices that promote complexation of Al with organic substances. Where knowledge about the functions of soil organic carbon is vital, it is only by studying the interaction of SOC with aluminium and soil components that is possible to understand its key impact in soil.
Since not all of ionic forms of Al are toxic to plants, determination of Al pools in soils and in aqueous systems within the natural environment using suitable extraction techniques is of utmost importance. Determination of extractable Al by the 1 M KCl method has been used as common standard extractant (Sposito, 2000). Although this is the standard method to measure "exchangeable" Al, this interpretation has been questioned for organic matter-rich soils and for soils where Al-humus complexes are abundant (Ponnete et al. 1996; Takahashi and Dahlgren, 1998). Soils from wetter regions of Portugal show large organic C contents and Al-humus complexes, and variable amounts of gibbsite in their clay fraction (Madeira and Furtado, 1987; Furtado et al., 1988; Martins et al., 1995). Therefore, the applicability of the aforementioned method should be compared with other extractants (such as 0.33 M LaCl3 and 0.5 M CuCl2), which have also been used as alternative extractants for Al associated with the organic matter and interlayer Al that influences the concentration of Al in soil solution (Oates and Kamprath, 1983; García et al., 2004). Monomeric species of Al have been successfully speciated in soil solution (Parker et al., 1995). Knowledge on the extractability of different forms of Al by chloride salts (i. e. 1 M KCl, 0.33 M LaCl3 and 0.5 M CuCl2) in the soil and on the concentration of different forms of Al in the soil solution is important for the management of Al effects in plants and for the determination of safe limits of Al concentration in the soil solution when liming soils.
Having this in view, a study was conducted to 1) determine the amounts of extractable Al in acidic soils derived from granite using the 1 M KCl, 0.33 M LaCl3 and 0.5 M CuCl2 extractants, 2) correlate the amounts of Al removed by different chloride salt extractants with soil properties and constituents, and 3) determine the dominant forms of Al in the soil solution.
MATERIALS AND METHODS
Soils
A total of twenty-two horizons (surface and subsurface), representing the range of soils derived from granite and occurring from dry subhumid to perhumid climate regions in Portugal (Table 1), were chosen according to their soil organic C content, clay and exchangeable Al (1 M KCl) contents and pH values (Table 2). Selected horizons have low pHH2O (4.5-5.3) values and contain different contents of organic C; kaolinite predominates in the clay fraction of soils in drier areas whereas in wetter areas gibbsitic materials can be the major components of such fraction (Madeira and Furtado, 1984). They also show low contents (0.07-3.18 cmolc kg-1) of exchangeable bases and extremely low saturation degree values (0.6-36%), especially those occurring in wetter areas. Study soils correspond to pedons located (Table 1) at Sapateiro (A), Cando (B), Gontim (C), Tribelo (D), Cabeças (E), Alto de Roçadas (F), Niza (G), Almeida (H), Montemuro (I), Castro Daire (J), and Serra da Estrela (K). All samples were air dried, mixed and sieved to 2 mm size prior to laboratory analysis.
Table 1 - Location and environmental conditions of study pedons. Alt- altitude; MAT- mean annual temperature; MAP- mean annual precipitation.
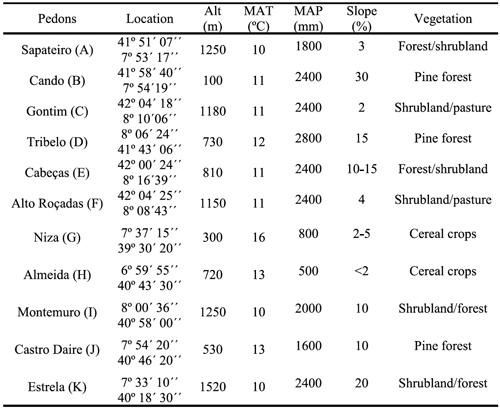
Table 2 - Values of pH, sum of bases (SB), cation exchange capacity (CEC), and contents of organic C (OC), clay (<2um), extractable Fe and Al by acid ammonium oxalate (Feo, Alo), bicarbonate-citrate-dithionite (Fed, Ald) and sodium pyrophosphate (Fep, Alp) in surface and subsurface horizons of studied pedons.
Analytical procedures
Soils were analyzed for pH in triplicate suspensions of 1 M KCl (1:2.5 soil: solution ratio) using a pH meter. Total soil organic C content of the soil samples was determined by wet oxidation following the Springer and Klee method (De Leenheer and Van Hove, 1958). Clay content was determined by pipette analysis following the dispersion method with sodium hexametaphosphate (Soil Survey Staff, 2004). Cation exchange capacity was determined using continuous leaching of 5 g soil with 100 mL of 1 M NH4OAc buffered at pH 7 (Soil Survey Staff, 1992). Basic cations (Ca2+, Mg2+, K+ and Na+) from the leachate were measured using atomic absorption spectrophotometer (Perkin Elmer Analyst 300), and sum of bases was calculated thereafter. Acid ammonium oxalate extractable aluminium (Alo) and iron (Feo), and Na-pyrophosphate extractable aluminium (Alp) and iron (Fep) were determined following the methods described by Blakemore et al. (1987), while Al (Ald) and Fe (Fed) extractable by dithionite-citrate-bicarbonate were determined following the procedures described by Mehra and Jackson (1960). Al and Fe from the filtered extracts were measured using the atomic adsorption spectrophotometer (Perkin Elmer Analyst 300) at 309 and 302 nm, respectively.
To evaluate the potential release of Al, successive extractions of Al were carried out in each sample. This was performed in duplicate by adding 5 g of soil in 50 mL plastic centrifuge tubes with 50 mL of chloride salt extractants (i.e. 1 M KCl, 0.33 M LaCl3 and 0.5 M CuCl2). Sample suspensions with 1 M KCl were shaken for 1 h, while those of 0.5 M CuCl2 and 0.33 M LaCl3 were shaken for 30 minutes using a reciprocal shaker. Samples were then centrifuged at 2500 rpm for 10 min and filtered using a Whatman no. 42 filter paper. The entrained solution left after centrifugation was corrected by weighing the tube and adding sufficient amount of the extractant for each sample to bring the volume of solution back to 50 mL. This extraction procedure was performed on each soil sample for five successive extractions. Aluminium in the extracts was measured by atomic absorption spectrophotometry at 530 nm. Cumulative Al released in the soil was computed by taking the total amount of Al released after five extractions.
The soil solution was extracted from 100 g soil (25 g of air dried soils in quadruplicate) packed uniformly in a Buchner funnel containing Whatman no. 542 filter paper. The soils were rewetted by adding the pre-determined volume of distilled water at field capacity (Adams et al., 1980). The moistened soils equilibrated for 24 h (Menzies and Bell, 1988) were then displaced slowly into Erlenmeyer flask, using a mechanical vacuum extractor, following the procedures described by Wolt and Graveel (1986). The pH of the soil solution was immediately determined on 2 mL sub-samples and Al in the soil solution was determined by the inductively coupled plasma atomic emission spectroscopy (ICPEAS). The amount of Al measured by the ICPEAS was regarded as total aluminium in the solution (AlT).
Inorganic monomeric aluminium (Alm) in the soil solution was determined by the modified aluminon method (Blamey et al., 1983), using the This method follow the same procedure as described by Hsu (1963) for total Al, but eliminates the acid digestion (i.e., addition of HCl and heating of the sample for 30 minutes in water bath at 80 ºC). The colour intensity was read 30 minutes after the addition of Al buffer solution. Values obtained were then compared to those determined by a short term pyrocatechol method of Kerven et al. (1989) to estimate the concentration of Alm. Organic Al in the solution was calculated as the difference between AlT and Alm.
Data analysis
Correlation and regression analyses of Al extracted by different chloride salts after the fifth extraction and Al forms in soil solution with soil constituents were determined using Statistica software (Statsoft, 2004).
RESULTS AND DISCUSSION
Extractability of Al
The amounts of Al extracted from study pedons by the 1 M KCl (Al-KCl), 0.33 M LaCl3 (Al-LaCl3) and 0.5 M CuCl2 (Al-CuCl2), following five successive extractions, are shown in the Table 3. After five extractions, the amounts of Al-KCl (0.93-8.74 cmolc kg-1) were consistently lower than those of Al-LaCl3 (1.70-11.86 cmolc kg-1), while those of Al-CuCl2 (2.43-30.86 cmolc kg-1) were higher than values of Al-LaCl3.
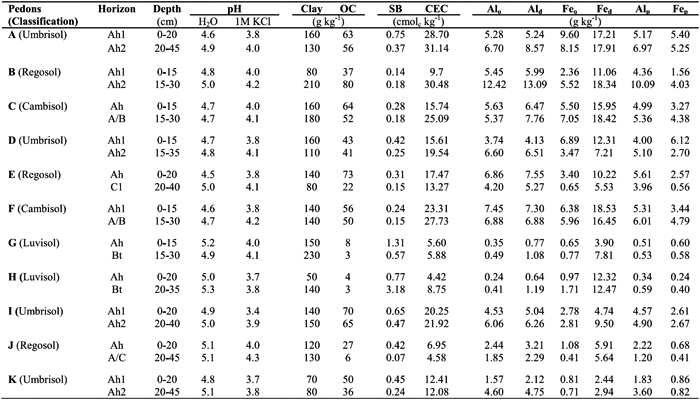
Table 3 - Amounts (cmolc kg-1) of Al extracted by the 1 M KCl (Al-KCl), 0.33 M LaCl3 (Al-LaCl3) and 0.5 M CuCl2 (Al-CuCl3) from surface and subsurface horizons of studied soils, following five repetitive extractions. Symbol: A (Sapateiro), B (Cando), C (Gontim), D (Tribelo), E (Cabeças), F (Alto de Roçadas), G (Niza), H (Almeida), I (Montemuro), J (Castro Daire) and K (Serra da Estrela).
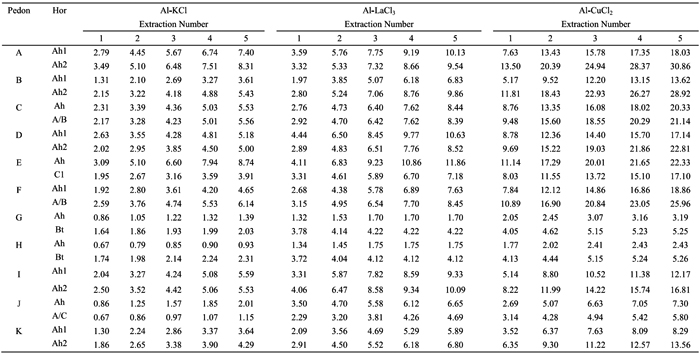
The amounts of Al-KCl showed a strong positive correlation with those of Al-LaCl3 (r=0.89, p<0.001) and of Al-CuCl2 (r=0.85, p<0.001); a positive correlation between the amounts of Al-LaCl3 and Al-CuCl2 (r=0.81, p<0.001) was also observed (Table 4). Considering all horizons of studied pedons, the values of Al extracted by the 0.33 M LaCl3 were 1-4 times higher than by the 1 M KCl, whereas values by the 0.5 M CuCl2 were 2-5 times higher (see Table 3).
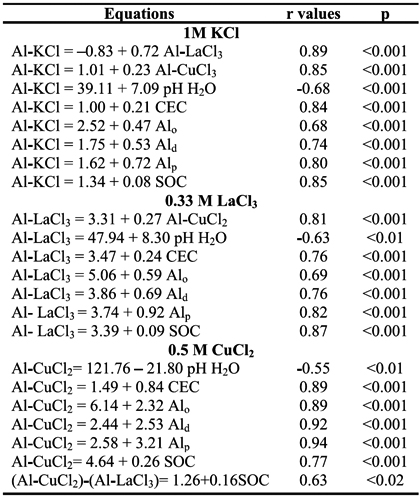
Table 4 - Relationships of Al extracted by the 1 M KCl (Al-KCl), 0.33 M LaCl3 (Al-LaCl3) and 0.5 M CuCl2 (Al-CuCl2) with soil characteristics and colloidal constituents.
The amounts of Al-KCl, Al-LaCl3 and Al-CuCl2 differed between surface and subsurface horizons. Subsurface Bt horizons of pedons G and H showed higher extractable Al contents than the surface horizons, which may be associated with higher clay content and, therefore, the higher cation exchange capacity in the former horizons. This trend was not observed in the subsurface horizons of pedons E and J (C1 and A/C, respectively), because they have similar clay content and much less organic C content than the surface ones. However, subsurface horizons (Ah2 or A/B) of other soils (pedons A, C, D, F, I and K), with similar clay content and lower organic C content than the surface ones (Ah1) showed higher contents of extractable Al, especially when the 0.5 M CuCl2 was used. This may be associated with a stronger interaction between soil organic matter (SOM) and aluminium in the subsurface horizons, which agrees with the higher Al amount extracted by the sodium pyrophosphate in such subsurface horizons (see Table 2). This also suggests that SOM characteristics may be different in the subsurface horizons, which is supported by the fact that a higher fulvic/humic acids ratio was reported by Madeira and Furtado (1984) for subsurface horizons of study soils occurring in wetter areas. We may also emphasizethat in the subsurface horizons, differences between amounts of Al-KCl and those of Al-CuCl2 were stronger (2-5 times) than in surface ones (2-4 times). Similar trend was observed between the amounts of Al-KCl and Al-LaCl3, 1-3 and 1-4 times, respectively in surface and subsurface horizons.
The low contents of Al-KCl measured in study horizons, as compared to those of Al-LaCl3 and Al-CuCl2 may reflect the ability of 1 M KCl to only extract the readily exchangeable Al, while the high contents of Al-LaCl3 and Al-CuCl2 may indicate their ability to extract both readily and non-readily exchangeable Al (that is, Al associated with organic matter, interlayer Al and hydroxyl-Al polymers) as reported by Bloom et al., (1979) and Oates & Kamprath (1983) for a wide range of acidic soils, and by García-Rodeja et al. (2004) for European volcanic soils. The ability of 0.5 M CuCl2 to extract higher amounts of Al from the soil may be associated with the strong power of Cu2+ ion to form complexes with the functional groups of organic matter in the soil (Matus et al., 2006). Additionally, the high efficiency of the CuCl2 to extract Al has been also explained by the acidic nature of the CuCl2 solution (pH 2.7-3.0) enhancing the greater release of Al from Al-organo complexes (Kaiser and Zech, 1996; Matus et al., 2008). On the other hand, La3+ ion showed a weaker ability to extract Al because it may extract Al from less hydroxylated and less polymerized Al species in organic matter (Hargrove and Thomas, 1981).
Positive significant (p<0.001) correlations between the contents of Al-CuCl2 and those of Alp, Ald and Alo (r=0.94, 0.92 and 0.89, respectively) were also observed, suggesting that Al extracted by the 0.5 M CuCl2 may be mostly associated with stable forms of Al and Al complexed with SOM. However, the correlation between the difference of Al extracted by the CuCl2 and the LaCl3 with the soil organic C (SOC) content was weaker (r=0.63, p<0.05), suggesting that the amount of Al specifically extracted by the CuCl2 may also be dependent on the SOM type. Although significant (p<0.001), correlations between Al-KCl and Al-LaCl3 contents, and Alp, Ald and Alo contents were weaker (r=0.80, 0.74, 0.68 and 0.82, 0.76, 0.69, respectively), indicating that Al extracted by these extractants is less associated with SOM. The relative effectiveness for displacing Al from horizons of studied pedons was Cu2+ > La3+> K+, which is in agreement with the findings reported by Bloom et al. (1979) and Oates and Kamprath (1983) for a wide range of organic soils. This indicated that the type of cation of the extractant has a major effect on the amount of Al extracted from the soil system and also governs the ability to remove Al in relation to the acidity regime that the cation created in a chloride solution (Oates and Kamprath, 1983).
The contents of Al-KCl, Al-LaCl3 and Al-CuCl2 showed significant negative correlation with pH in H2O (r=-0.68, p<0.001, -0.63, p<0.01 and -0.55, p<0.01, respectively), while correlations with pH in 1 M KCl were not significant (p>0.05) (see Table 4). In fact, according to Oates and Kamprath (1983), readily exchangeable Al and Al-organo complexes are made less available from exchange sites at pH > 5.5. A strong positive correlation with the amounts of Al-KCl, Al-LaCl3 and Al-CuCl2, and values of CEC (r=0.84, p <0.001, 0.76, p<0.001 and 0.89, p<0.001, respectively) was also observed (Table 4), indicating the essential role of charged components, mainly from soil organic acids, on the release of Al from Al-organo complexes (García et al., 2004).
A substantial variation in the amounts of Al removed by the studied salt extractants was noted in accordance with the SOC contents (Figure 1). In fact, the surface and subsurface horizons of pedons G, H and J (sites from Niza, Almeida and Castro Daire, respectively), subsurface horizon (C1) of pedon E (sites from Cabeças), containing low SOC content (3-27 g kg-1) showed the lowest ranges of Al-KCl (0.9-3.9 cmolc kg-1), Al-LaCl3 (1.7-7.2 cmolc kg-1) and Al-CuCl2 (2.4-17.1 cmolc kg-1). In contrast, the surface and sub-surface horizons of other study pedons, containing high contents of SOC (36-80 g kg-1), showed higher ranges of Al-KCl (3.6-8.7 cmolc kg-1), Al-LaCl3 (5.9-11.9 cmolc kg-1) and Al-CuCl2 (8.3-30.9 cmolc kg-1) than in the aforementioned soils. This is in conformity with the strong positive correlation of SOC content with the contents of Al-KCl, Al-LaCl3 and Al-CuCl2 (r=0.85, p < 0.001; 0.87, p < 0.01; and 0.77, p < 0.01, respectively) (Fig. 1), emphasizing the role of SOC contents in soil extractable Al.
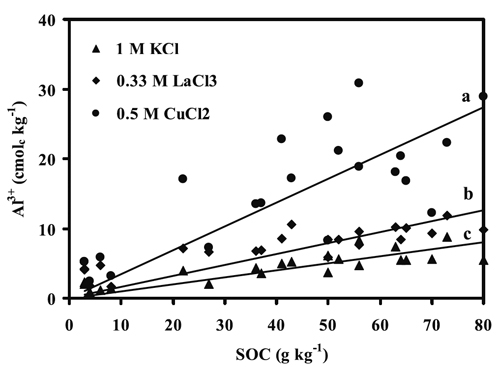
Figure 1 - Relationships between the amounts of Al3+ extracted by chloride salts and soil organic C (SOC) content in studied horizons. a: 0.5 M CuCl2 = 4.64 + 0.26 SOC (r = 0.77, p < 0.001); b: 0.33 M LaCl3 = 3.39 + 0.09 SOC (r = 0.87, p < 0.001); c: 1 M KCl =1.34 + 0.08 SOC (r = 0.85, p < 0.001).
The low amounts of Al removed by the extractants from pedons G, H and J may be ascribed to low SOC content, which also coincides with a low range of mean annual precipitation (500-1600 mm) and therefore to conditions less favourable for leaching (Table 1). In fact, a positive correlation between SOC content and mean annual precipitation was reported for similar conditions in Portugal (Martins et al., 1995). Our results suggest that the high accumulation of organic matter (organic C contents of 36-80 g kg-1) and pH decrease, under rainy conditions (mean annual precipitation of 1600-2400 mm) (Martins et al., 1995), may have contributed to dissolution of Al from gibbsite, leading to the formation and stabilization of Al-organo complexes as reported by Madeira & Furtado (1987) and Madeira and Furtado (1984). This is also in conformity with the results reported by Wilke and Schwertmann (1977) for strongly acid soils from Baviera. However, Al complexed by organic ligands may be partially maintained in solution and may then be transported into lower horizons of the soil profile or lakes and streams (Lindsay and Walthall, 1989).
Our results also indicate that accumulation and stabilization of organic matter in the study soils may be associated with interactions with active noncrystalline (amorphous or poorly crystalline inorganic) and organically complexed forms of Al as stressed by Madeira and Furtado (1984).
Forms of Al in the soil solution
The concentrations of total (AlT), monomeric (Alm) and organic (Alorg) Al in the soil solution of studied pedons are shown in the Figure 2. As observed for Al extracted from soil by the different chloride salt extractants, the concentrations of AlT, Alm and Alorg also varied according to soil horizons, SOC contents (Table 2) and differences regarding environmental conditions where the soils developed (Table 1). Concentration variations were much stronger for Alorg than for Alm.
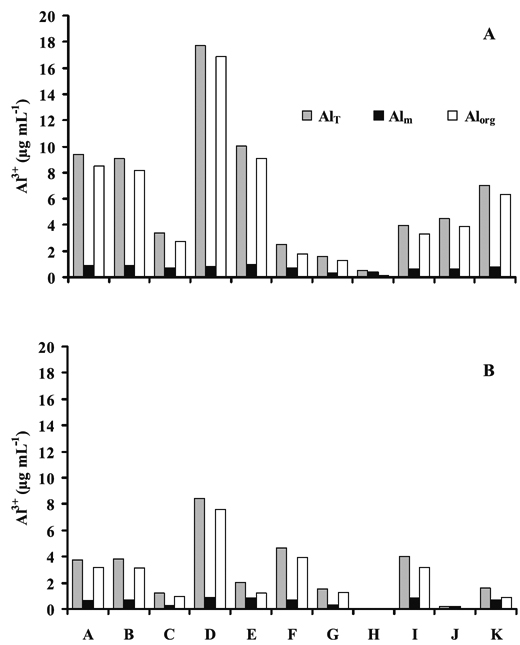
Figure 2 - Mean concentrations of total Al (AlT), monomeric Al (Alm) and organic Al (Alorg) in the soil solution (µg mL-1) from surface (A) and subsurface (B) horizons of studied pedons. A (Sapateiro), B (Cando), C (Gontim), D (Tribelo), E (Cabeças), F (Alto de Roçadas), G (Niza), H (Almeida), I (Montemuro), J (Castro Daire) and K (Serra da Estrela).
Surface and subsurface horizons of areas with mean annual precipitation less than 1000 mm and low organic C content (pedons G and H) showed the lowest range of AlT, Alm and Alorg concentrations in soil solution (0.09-1.60, 0.06-0.38 and 0.03-1.29 µg mL-1, respectively), while concentrations in soils in wetter areas and with higher organic C content (e.g. pedons A, B, E, D, and K) were 7.1-17.7,0.8-0.9 and 6.3- 16.9 µg mL-1, respectively.
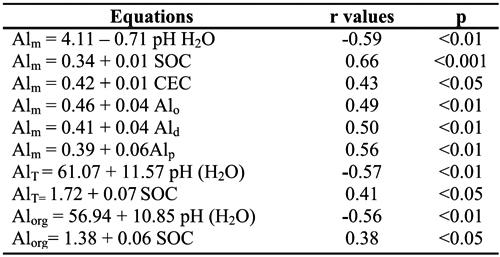
Values of Alm showed negative correlation with soil pH in water (r=-0.59, p < 0.01) and positive correlations with the values of CEC (r=0.43, p < 0.05) and contents of Alo, Ald and Alp (r=0.49, 0.50, 0.56, p < 0.01, respectively) (Table 5); concentrations of Alm were also positively correlated with values of Al-K (r=0.609, p<0.01), Al-La (0.694, p<0.001) and Al-Cu (r=0.530, p<0.01). Values of AlT (r=0. 577, p<0.01) and Alorg (0.565, p<0.01) also showed negative correlations with soil pH in water. Correlation of Alm values with the soil organic C content was positive and stronger (r=0.664, p<0.001) than that observed for Alorg (0.38, p<0.05) and AlT (0.41, p<0.05). Alorg values were also correlated with Al-K (r=0.441, p<0.05) and Al-La (0.574, p<0.01) contents.
Table 5 - Relationships of monomeric (Alm), organic (Alorg) and total (AlT) Al in soil solutionwith soil pH in H2O and soil colloidal constituents.
Overall, our data showed that in both surface and subsurface horizons the concentrations of Alorg were much higher than those regarding Alm. For instance, Alorg concentrations in surface horizons (except pedon H) were 2-21 times higher than those of Alm; in subsurface horizons (except pedons H and J) they were 1-9 times higher. Differences were much larger in soils located in wetter areas than in the others; that is, were larger in soils with higher organic C content. This further suggests that the concentration of Alorg is the largely dominant form of Al in the solution of studied soils. Concentration of Alorg, which showed a strong correlation with AlT (0.99, p<0.001), may be a good indicator of the organic C complexes with Al in the soil solution, and thus may contribute to the reduction of potential Al toxicity (Haynes and Mokolobate, 2001). In fact, organically complexed forms of Al in soil solutions and in natural waters are reported to be much less toxic to plants and aquatic systems than Al3+ or its hydrated monomers (Stevenson & Vance, 1989). Our results are in agreement with the fact that organic complexed Al can represent a considerable proportion of soluble Al in surface waters (Driscoll et al., 1983). Also, it corroborates with the high proportions of organic complexed Al in Podzol B horizons leachates and its linear correlation with organic C in solution, as reported by Nilsson and Bergkvist (1983).
Despite the influence of SOM content on the amount of extractable Al, we may emphasise that Alorg in soil solution of study soils showed a weak correlation with SOC contents (Table 5); also, values of the ratio of Alorg in surface and subsurface horizons were not correlated with organic C content (p>0.05). In fact, low proportions of Alorg (in relation with AlT) were observed in the present study (25-33%) for the pedon H, with very low contents of organic C (3-4 g kg-1), suggesting that low proportions of Alorg may be associated with low soil organic C contents. However, pedon G, occurring in a subhumid area and with low soil organic C contents (3-8 g kg-1) showed much higher proportions of Alorg in the soil solution (80%). Such trend may be associated with differences on the nature of the SOM and its quality (namely soluble fractions), and land use system. That is, it may be more related to dynamics of SOM than with its content in the soil.
Our results also suggest that low proportions of Alorg in the soil solution of subsurface horizons may be associated with lower organic C content. In fact, in the subsurface horizon of pedon J, occurring in a wetter area (mean annual precipitation of 1600 mm), but with a low organic C content (6 g kg-1), the lowest Alorg proportion (6%) was observed.
Pedons C and F, occurring in wetter areas and with high organic C contents, showed much lower AlT and Alorg concentrations than soils of similar areas (Figure 2); also, the proportion of Alorg was lower (71-80%) than in the others (83-95%). As pedons C and F occur in shrubland areas and the others in forest or forest/shrubland, we may speculate that such differences could be also associated with the type of vegetation cover.
High concentrations of Al measured in soil solution of studied soils are in accordance with the very high Al saturation of the respective exchangeable complex, that is, with the extremely low degree of base saturation. Such high concentrations (1-18 mg L-1) and levels of Al saturation may apparently cause strong toxicity effects (Sanchez, 1986). However, a large proportion of Al in soil solution is associated with organic matter (monomeric Al usually less than 1 mg L-1), and therefore limiting its potential toxicity effects. In fact, presence and addition of organic compounds are considered often useful in complexation with Al, thus reducing its potential toxicity (Grathwohl, 1990). As organic matter reactions with Al and Al-bearing minerals have been reported to protect soils from reaching toxic soluble metal concentrations of Al (Krull et al., 2005), we emphasize that Al toxicity in most of studied soils may be restricted by their high organic matter content.
CONCLUSIONS
Extractability of Al in soils developed on granitic rocks was in the order: CuCl2 >LaCl3 >KCl. Amounts of Al extracted by these extractants were negatively correlated with soil pH in water and positively correlated with soil organic C content. Strong correlations between extractable Al and contents of noncrystalline (amorphous or poorly crystalline inorganic and organically bound) forms of Al were observed, being stronger in the case of Al extracted by 0.5 M CuCl2. Al amounts extracted by studied extractants were markedly higher in soils containing large amounts of organic carbon than in the others. Concentrations of organic (Alorg) aluminium were largely predominant in the soil solution especially in soils occurring in wetter areas with higher contents of organic C. Monomeric and organic Al were weakly correlated with soil organic C content. The activity of Al in soil solution of studied soils maybe mostly governed by the content of soil organic C. Further evaluation on the changes in the concentration of different forms of Al and its activity in the solution after liming should be developed.
REFERENCES
Adams, F.; Burmester, C.; Hue, N.V. and Long, F.L. (1980) - A comparison of column-displacement and centrifuge methods for obtaining soil solutions. Soil Sci. Soc. Amer. Proc., 44: 733-735. [ Links ]
Auxtero, E.; Madeira, M. and Sousa, E. (2004) - Variable charge characteristics, and cation and anion exchange properties of selected cambisols derived from granite in Portugal. Revista de Ciências Agrárias, 27, 1: 49-62. [ Links ]
Barnhisel, R. and Bertsch, P. (1982) - Aluminium. In: Page, A.L. (Ed.) - Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Madison, USA, Inc. and Soil Science Society of America, Inc., p. 275-300. [ Links ]
Blakemore, L.C.; Searle, P.L. and Daly, B.K. (1987) -Soil Bureau Laboratory Methods: A Method for Chemical Analysis of Soils. New Zealand, Soil Bureau Scientific Report,80. [ Links ]
Blamey, F.P.C.; Edwards, D.G. and Asher, C.J. (1983) - Effects of aluminium OH:Al and P:Al molar ratios and ionic strength on soybean root elongation in solution culture. Soil Sci., 136, 4: 197-207. [ Links ]
Bloom, R.P.; Mcbride, M.B. and Weaver, R.M. (1979) - Aluminum organic matter in acid soils: buffering and solution aluminium activity. Soil Sci. Soc. Am. J., 43: 488-493. [ Links ]
De Leenheer, L. and Van Hove, J. (1958) - Determination de la teneur en carbone organique des sols. Études critiques des metodes tritrimétriques. Pédologie, 8: 39-77. [ Links ]
Fripiat, J.J. and Herbillon, A.J. (1971) - Formation and transformations of clay minerals in tropical soils. In: Soils and Tropical Weathering. Proc. of Bandung Symposium. Paris, UNESCO, p. 15-24. [ Links ]
Furtado, A.F.S. and Madeira, M.A.V. (1988) - Ocorrência da gibsite em solos derivados de granitos em Portugal Continental. II Congresso Nacional de la Ciencia del Suelo, 26-30 de Septiembre, Sevilla, p. 535-541. [ Links ]
García-Rodeja, E.; Nóvoa, J.C.; Pontevedra, X.; Martínez-Cortizas, A. and Buurman, P. (2004) - Aluminium fractionation of European volcanic soils by selective dissolution techniques. Catena, 56: 155-184. [ Links ]
Grathwohl, P. (1990) - Influence of organic matter from soils and sediments from various origins on the sorption of some chlorinated aliphathic hydrocarbons: implications on KOC correlations. In: Krull, E.S., Skjemstad, J.O. and Baldock, J.A. (Eds.) - Functions of soil organic matter and the effect on soil properties. Residue Management, Soil Organic Carbon and Crop Performance. Grains Research and Development Corporation Project no. CSO 00029, 129 p. [ Links ]
Haynes, R.J. and Mokolobate, M.S. (2001) - Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: a critical review of the phenomenon and the mechanisms involved. Nutrient Cycling in Agroecosystems, 59: 47-63. [ Links ]
Hsu, N.V. (1963) - Effect of inicial pH, phosphate and silicate on the determination of aluminium with aluminon. Soil Sci., 96: 230-238. [ Links ]
Kaiser, K. and Zech, W. (1996) - Defects in estimation of aluminum in humus complexes of podzolic soils by pyrophosphate extraction. Soil Sci., 161: 452-458. [ Links ]
Kerven, G.L.; Edwards, D.G.; Asher, C.J.; Hallman, P.S. and Kokot, S. (1989) - Aluminium determination in soil solution. 2. Short term colorimetric procedures for the measurement of inorganic monomeric aluminium in the presence of organic acid ligands. Aust. J. Soil Res., 27, 1: 91-102. [ Links ]
Krull, E.S.; Skjemstad, J.O. and Baldock, J.A. (2005) - Functions of soil organic matter and the effect on soil properties. Residue Management, Soil Organic Carbon and Crop Performance. Grains Research and Development Corporation Project no. CSO 00029, 129 p. [ Links ]
Lindsay, W.L. and Walthall, P.M. (1989) - The solubility of aluminium in soils. In Sposito, G. (Ed.): - The environmental chemistry of aluminum. Florida: CRC Press, Inc., p.221-239. [ Links ]
Madeira, M. and Furtado, A.F.A.S. (1984) - Os solos formados a partir de rochas graníticas sob clima temperado super-húmido (Parque Nacional da Peneda-Gerês). Suas características mais relevantes. Anais do Instituto Superior de Agronomia, 41: 9-54. [ Links ]
Madeira, M. and Furtado, A.F.A.S. (1987) - The instability of gibbsite and occurrence of other aluminous products in soils of perhumid climate regions of Portugal. Garcia de Orta, Série Geologia, 10, 1-2: 35-41. [ Links ]
Martins, A.; Madeira, M. and Réffega, A. (1995) - Influence of rainfall on properties of soils derived from granite in Portugal. Arid Soil Res. Rehab., 9: 353-366. [ Links ]
Matus, F.; Amigo, X. and Soren, K.M. (2006) - Aluminium stabilization controls organic carbon levels in Chilean volcanic soils. Geoderma 132: 158-168. [ Links ]
Matus, F.; Garrido, E.; Sepúlveda, N.; Cárcamo, I.; Panichini, M. and Zagal, E. (2008) - Relationship between extractable Al and organic C in volcanic soils of Chile. Geoderma,148: 180-188. [ Links ]
Mehra, B.P. and Jackson, H.L. (1960) - Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium carbonate. Clays and Clay Min., 7: 317-327. [ Links ]
Menzies, N.W. and Bell, L.C. (1988) - Evaluation of the influence of sample preparation and extraction technique on soil solution composition. Aust. J. Soil Res., 26:451-464. [ Links ]
Nilsson, S.I. and Bergkvist, B. (1983) - Aluminium chemistry and acidification processes in shallow podzol on the Swedish westcoast. Water Air Soil Pollut., 20: 311-329. [ Links ]
Oates, K.M. and Kamprath, E.J. (1983) - Soil acidity and liming: Effects of the extracting solution cations and pH on the removal of aluminium from acid soils. Soil Sci. Soc. Am. Proc., 47: 686-689. [ Links ]
Parker, D.R.; Zelazny, L.W. and Kinraide, T.B. (1995) - Improvement to the program GEOCHEM. Soil Sci. Soc. Am. J., 5: 488-491. [ Links ]
Pereira, V. and Fitzpatrick, E.A. (1995) - Cambisols and related soils in north-central Portugal: their genesis and classification. Geoderma, 66: 185-212. [ Links ]
Ponette, Q.; Andre, D. and Dufey, J.E. (1996) – Chemical significance of aluminium extracted from three horizons of an acid forest soil, using chloride salt solutions. European J. Soil Sci., 47: 89-95. [ Links ]
Sanchez, P. A. (1986) - Properties and management of soils in the tropics. New York, John Wiley and Sons. [ Links ]
Soil Survey Staff (1992) - Soil survey laboratory methods manual. Washington, D.C., USDA-NRCS [ Links ]
SSS (Soil Survey Staff). (2004) - Soil survey laboratory methods manual. Soil survey investigations rep., vol. 42, Washington, D.C., USDA-NRCS. [ Links ]
Sposito, G. (2000) - The environmental chemistry of aluminum. Florida, CRC Press, Inc. [ Links ]
Statsoft, (2004) - A division of Statsoft Iberica, Inc. Lisbon, Portugal. [ Links ]
Stevenson, F.J. and Vance, G.F. (1989) - Naturally occurring aluminum-organic complexes. In:Sposito, G. (Ed.) - The environmental chemistry of aluminum. Florida, CRC Press, Inc. p.117-145. [ Links ]
Takahashi, T. and Dahlgren, R.A. (1998) - Possible control of aluminium solubility by 1 M KCl treatment in some soils dominated by aluminium-humus complexes. Soil Science and Plant Nutrition, 44: 43-51 [ Links ]
Wilke, B.M. and Schwertmann, U. (1977) - Gibbsite and halloysite decomposition in strongly acid podzolic soils developed from granite saprolite of the bayerischen wald. Geoderma, 19: 51-61. [ Links ]
WRB (World Reference Base for Soil Resources) (2006) - A framework for international classification, correlation and communication. World soil resources reports, 103. Rome, FAO. [ Links ]
Wolt, J. and Graveel, J.G. (1986) - A rapid routine method for obtaining soil solution using vacuum displacement. Soil Sci. Soc. Amer., J. 50: 602-605. [ Links ]
ACKNOWLEDGEMENTS
The first author is grateful to the Fundação para a Ciência e Tecnologia for supporting this post-doctoral research (2008-2011). Authors thank Dra. Fátima Calouro, Director of the Laboratório Química Agrícola Rebelo da Silva, for the use of inductively coupled plasma atomic emission spectroscopy (ICPEAS), Natália Correia and Adozinha Curta, for assistance in the use of ICPEAS, and technicians of the Soil Laboratory of the Instituto Superior de Agronomia, for technical assistance.
Recepção/Reception: 2011.03.21
Aceitação/Acception: 2011.03.29














