Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Ciências Agrárias
Print version ISSN 0871-018X
Rev. de Ciências Agrárias vol.40 no.3 Lisboa Sept. 2017
https://doi.org/10.19084/RCA16133
ARTIGO
Rice growth under water stress levels imposed at distinct developmental stages
Crescimento do arroz sob níveis de estresse hídrico impostos em diferentes estádios de desenvolvimento
José Maria Barbat Parfitt1, Germani Concenço1,*, Kelly Downing2, Jake Larue2 e Jaqueline Trombetta da Silva3
1Empresa Brasileira de Pesquisa Agropecuária, Embrapa Clima Temperado, Pelotas, RS, Brazil
2Valmont Industries, Irrigation Division, Valley-NE, USA
3Department of Soil Science, Graduate Program in Management and Conservation of Soil and Water, Universidade Federal de Pelotas [UFPEL], Pelotas, RS, Brazil
(*Email: germani.concenco@embrapa.br)
SUMMARY
We aimed to assess rice growth as function of water stress at distinct crop stages, under greenhouse, using randomized block design and factorial scheme 3 × 4 + 1, with four replications. Factor A was the growth stage when water stress was imposed, being (1) vegetative, (2) reproductive 1, and (3) reproductive 2; factor B was four levels of water stress (0 ‑ 200 kPa). The additional treatment consisted of a flooded check. Water was replenished to saturation every time the threshold stress was reached. Rice height is reduced when stress is applied either at vegetative or reproductive stages; tillering was affected only by water stress at the vegetative stage, and plants did not recover after the stress was withdrawn; surplus in leaf area is likely to help recovering plant performance from stress applied at the vegetative stage; water content was not affected by water stress, but shoot dry mass of culms was reduced with stress between tillering start and anthesis; root volume did not increase as function of water stress at the vegetative stage. Rice growth is little affected by the absence of waterlogging, but stress levels should be below 30 kPa throughout cropping cycle.
Keywords: Oryza sativa, phenology, water demand.
RESUMO
Avaliou-se o crescimento do arroz em função do estresse hídrico em diferentes fases fenológicas, em casa‑de‑vegetação, em delineamento de blocos casualizados, esquema fatorial 3 × 4 + 1, com quatro repetições. O fator A representou a fase fenológica da planta com imposição de estresse, sendo (1) vegetativo, (2) reprodutivo 1, (3) reprodutivo 2; o fator B representou os níveis de estresse (0 ‑ 200 kPa); o tratamento adicional foi composto por uma testemunha inundada. A água foi reposta ao alcançar o nível de estresse determinado para o tratamento. As plantas cresceram menos com estresse tanto nas fases vegetativa como reprodutiva; o perfilhamento foi afetado pelo estresse na fase vegetativa; a expansão de área foliar auxilia na posterior recuperação da planta quando o estresse foi imposto na fase vegetativa; o conteúdo de água não foi afetado pelo estresse, mas a massa seca de colmos foi reduzida quando o estresse ocorreu entre início do perfilhamento e antese; por fim, o volume de raízes não aumentou com imposição de estresse na fase vegetativa. O crescimento do arroz é pouco afetado pela falta de inundação, mas o estresse hídrico deve permanecer abaixo de 30 kPa durante todo o ciclo da cultura.
Palavras-chave: Oryza sativa L., fenologia, demanda hídrica.
INTRODUCTION
Rice is a staple food for nearly half the world's population, being cultivated in 112 countries, with 90% of the worlds production concentrated in Asia. In Brazil, about 3 million hectares are cultivated every year and rice is traditionally present in Brazilian meals, regardless of social class. The southern region of the country supplies approximately 65% of Brazilian rice (Gomes and Magalhães Jr., 2004). The demand for water in flooded rice cultivation is considerably higher than the water requirement of crops traditionally sprinkler irrigated, such as soybeans and corn. This raises a series of questions, ranging from water use efficiency to environmental issues when growing rice. Physiologically, this species is a sub-aquatic plant adapted to a flooded environment (Correll and Correll, 1975), being thus possible to be grown in absence of waterlogging.
Sprinkler irrigation by pivot and linear system has been, and continues to be, tested for rice cultivation, and there are claims for 50% water savings when rice grown under pivot irrigation is compared to continuous flooding (Parfitt et al., 2011). This seems to be the case mainly when the system is installed in uneven areas or in fields with significant slope, as well as, where water is scarce. Supposing this economy is confirmed, farmers who grow rice under pivots would have a surplus of water that could be used either to increase rice acreage or to irrigate crops on additional fields.
In order to support rice-growing systems which demand less water and help environmental conservation, there is a need to understand how rice plants are affected by, and respond to, distinct levels of water stress imposed at different crop phenological stages. This knowledge is the base, which paves the way for the planning of proper, low-cost and efficient rice production systems while reducing the environmental impact in its production.
Water content in plants is important for keeping hydration, maintaining cell turgidity for structure and growth; it is also greatly responsible for the transport of nutrients and organic compounds into the plant, and also serves as raw material for chemical processes and buffers the plant against temperature oscillations (Gurevitch et al., 2009). As rice is usually grown under waterlogged soils, there was little need in the past to understand the effects of drought in rice development. When rice passed to be grown under sprinkler irrigation in highland soils, water stress started to be significant.
Besides water content, rice plant height, tillering vigor and leaf area are major factors responsible for its competitiveness against important weed species (Aldrich, 1984). For a crop to become successful, it should germinate and emerge fast after planting, and occupy the area as rapidly as possible to avoid the establishment of other competitor plants (Gurevitch et al., 2009).
This study aimed to assess rice growth parameters as function of water stress levels imposed at distinct stages of crop development.
MATERIALS AND METHODS
The experiment was installed in a greenhouse at Embrapa Clima Temperado, Pelotas-RS, Brazil, during the traditional rice-growing season. A randomized complete-block design with plots arranged in a factorial scheme, 3 × 4 + 1, with four replications was used. The rice variety was BRS-Querencia, with early cycle duration (Embrapa, 2005). Factor A comprised the growth stage when water stress was imposed on the treatments, being (1) vegetative (from the beginning of tillering to panicle differentiation), (2) reproductive 1 (from panicle differentiation to anthesis), and (3) reproductive 2 (from anthesis to the beginning of ripening). Factor B comprised the four levels of water stress imposed to the plants. The additional treatment consisted of a constantly flooded treatment (Table 1).

Although the reproductive stage in rice starts at panicle initiation (SOSBAI, 2016), this stage is very difficult to identify; as a result, farmers generally use panicle differentiation as the start of the reproductive stage for nutrient management. As panicle initiation (PI) and panicle differentiation (PD) are spaced only in about four days (Carli et al., 2016), panicle differentiation was used in the present study.
From emergence to the beginning of tillering, all plots were maintained with soil water tension less than 10 kPa - including plots which would be flooded from tillering onward. Every time the treatment reached the threshold level of water deficit, it was irrigated back to saturation. Treatments that were not at the developmental stage when the stress was previewed to be applied were maintained under 10 kPa. Treatments are listed in Table 1.
Experimental units consisted of black plastic pots with a capacity of 12 L, filled with 10 kg of previously corrected and fertilized soil. The soil used at the experiment was collected in agriculture-free natural areas near rice fields at Terras Baixas Experimental Station, Capão do Leão, RS, Brazil. Soil was fertilized with N-P-K and corrected for pH 6.0 with ground limestone. In rice fields, pH is usually not corrected because the water layer is enough to correct the pH after flooding is established, but as most plots of the trial were not going to be submitted to flooding, a correction of soil pH was done in order to guarantee equal soil pH conditions for all plots. Seven rice seeds were sown into each experimental unit, and after emergence the five most homogeneous plants were maintained.
Water stress was monitored by using sets of Watermark (model 200SS) electro-tensiometers (Irrometer Inc., Riverside, CA), with a single sensor installed in each experimental unit, at depth of 10 cm (from soil surface to the center of the sensor), at the radial center of the pot. All sensors were connected by wire to a nearby Watermark data logger, which was programmed to record water tension in kPa at one-hour intervals. The data logger automatically corrected sensor readings as a function of the mean temperature registered inside the plots, and for that, two Watermark temperature sensors were installed in each block of the experiment. Temperature data from these sensors were used by the data logger to correct soil water tension readings of the corresponding plots. Temperature sensors were also installed at a depth of 10 cm.
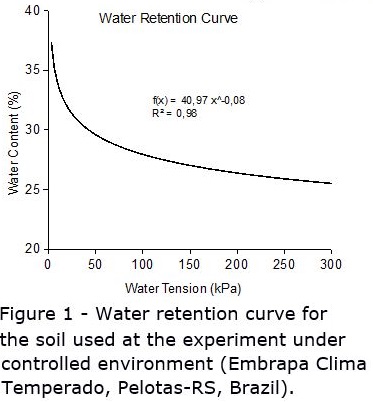
Soil water tension for all plots was read and recorded manually, twice a day (09:00 am and 04:00 pm), seven days a week. When it reached the threshold level, the water needed to adjust soil water tension back to saturation was added after reading. The amount of water to be added to each plot was determined by using a soil moisture retention curve, which relates water tension (kPa) with water content (%). The water tension curve was determined especially for the experiment (Figure 1), after the soil was corrected and fertilized, so no error in the curve would be attributed to differential soil density or structure.
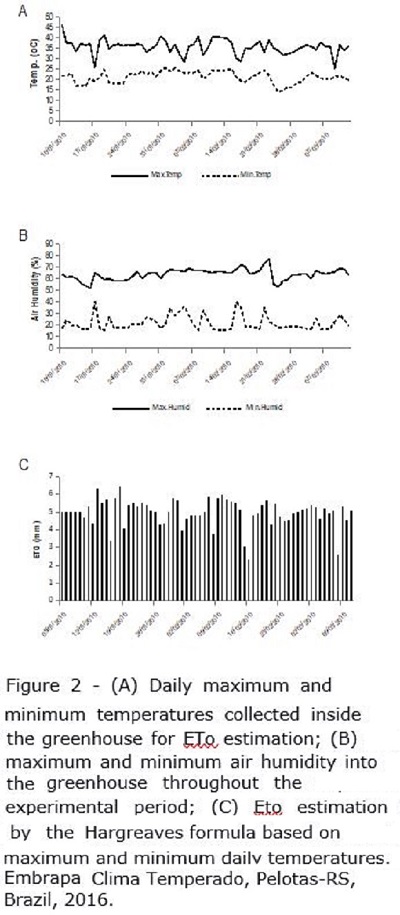
The daily maximum and minimum temperature and air humidity into the greenhouse during the application of the treatments are shown in Figure 2A and 2B, respectively. The evapotranspiration (ET0) was calculated as an auxiliary method to the sensors supporting estimation of when plots would reach the threshold water stress level in order to avoid losing the timing for water application. ET0 was calculated using the Hargreaves equation (Figure 2C), based on daily maximum and minimum temperatures (Figure 2A).
Weekly, starting from seven days after treatment establishment to the V group, the plant height, number of tillers per plant and leaf area were assessed. Plant height was evaluated by using a graduated ruler measuring the distance from the soil surface to the tip of the taller leaf; to access the number of tillers, tillers were counted in all plants, being presented as mean number of tillers per plant; the leaf area was not directly measured but estimated, as this is a destructive parameter when directly read. For that, the length and width of all leaves per plant were measured, being the estimated leaf area obtained by the following formula (Carlesso et al., 1998):
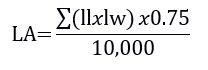
where LA= (estimated) leaf area (m2); ll = leaf length (cm); lw = leaf width (cm).
At the end of the cycle, rice shoot water content (%), dry mass (g plant-1) and root volume (cm3 plant-1) were also assessed. Plants were cut at soil level, immediately weighed for fresh mass being later dried in air-circulated oven at 65 ºC for four days, when the dry mass was evaluated. Shoot water content (%) was obtained by the following formula (Minshall, 1960):
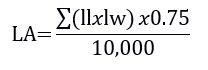
where WC = water content (%); FW = fresh weight; DW = dry weight.
After collecting the plants, all the soil of each experimental plot was carefully washed under running water for obtaining the bare roots; the excess of water in roots was removed with absorptive paper towels by gently pressing and rubbing them against the roots until towels were almost dry after used. Roots were put into a graduated tube containing water, being root volume obtained by the difference between the water level before and after root immersion.
Data were analyzed into the R statistical environment (R Core Team, 2016). Before any analysis, data sets were verified for normality and variance homogeneity of residuals by the tests of Shapiro-Wilk and Bartlett, respectively, being transformed by √(x+1) when needed. Data were then submitted to analysis of variance by the F-test at 5% probability. Data were explored according to significances of the interactions, being presented in graphical form. Data graphically presented are original; transformed data were used only for the parametric tests.
RESULTS
Plant height (Figure 3) was affected by water stress when it was imposed both at V and R1 stages; as rice plants stop growing before R2, differences due to stresses imposed at this stage were not expected. For the V stage, plants tended to be lower than the observed for the flooded check when water stress was beyond 100 kPa; 28 days after stress was imposed (after tillering start - DAT), plants at the two higher water tensions measured about 40cm height, while those at the flooded check and also those whose water stress was up to 30 kPa, measured about 60 cm (Figure 3). The stress for the V stage was maintained for 30 days, and 42 DAT (12 days after stress was removed from V stage), plant height was recovered and no difference among treatments was later reported.
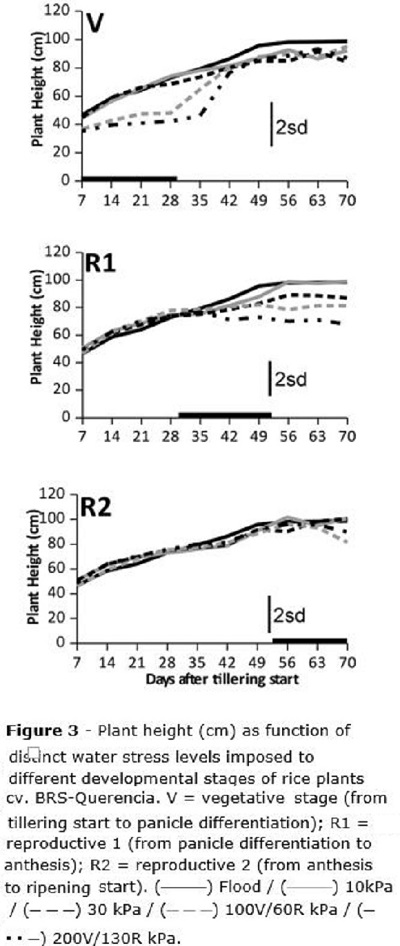
For R1, only the stress level of 130 kPa caused reduction in plant height compared to the flooded check (Figure 3), and this was maintained to the end of the cycle; no growth recovery was observed when severe water stress was imposed between panicle differentiation and anthesis (R1). When stress was imposed at R2, no damage to plant height was reported, since plants had already stopped growth at all.
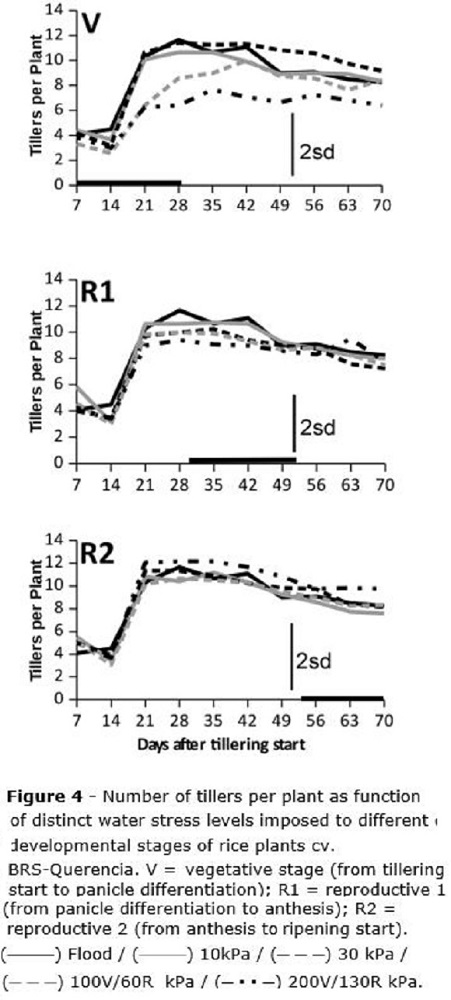
The number of tillers per plant was also affected by the water stress, but only at the V stage (Figure 4), when water tensions of 100 and 200 kPa reduced tillering, respectively, in about 30 and 48%. Under 100 kPa, tillering was smoothly recovered after stress was removed, while for 200 kPa no recovery was observed in tillering after the stress was stopped (Figure 4). At the V stage, for the flooded check as well as for treatments until 30 kPa, only the usual tiller mortality by intraspecific competition was reported (Figure 4), being the same observed for the R1 and R2 stages. Considering the usual tiller mortality by competition, a maximum of 11-12 tillers was reported per plant, being reduced to 8-9 tillers per plant at the end of the cycle (Figure 4).
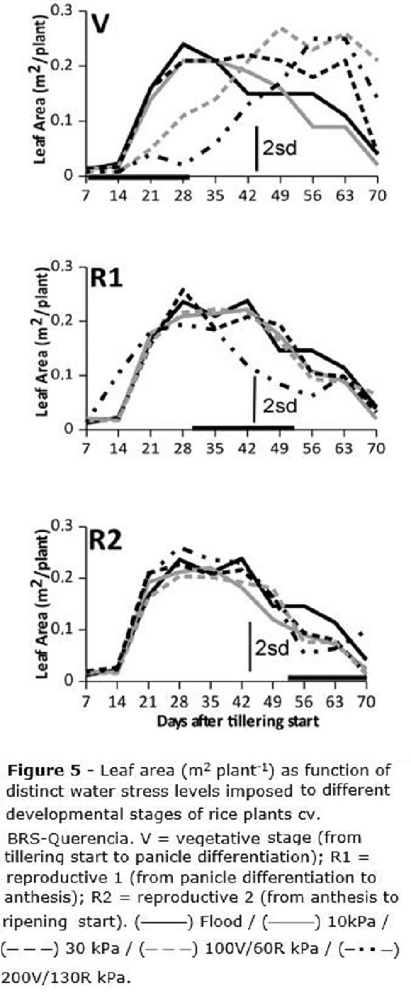
Leaf area (Figure 5) was about 200-380 cm2 per plant (0.02-0.038 m2 per plant) for all treatments until seven DAT (about 18 days after emergence - DAE), after this period it started growing fast and the maximum leaf area was observed about 28 DAT / 39 DAE, reaching about 0.18-0.25 m2 plant-1. Leaf area increase was halted for stress levels above 100 and 130 kPa, for V stage and above 130 kPa for R1 stage (Figure 5); leaf area was slowly recovered after the stress was removed. For the R2 stage no leaf area reduction was observed for any water stress level (Figure 5). For any treatment whose stress level did not cause leaf area restrictions, the leaf area curve was the usual, e.g., it slowly decreased after reaching a peak.
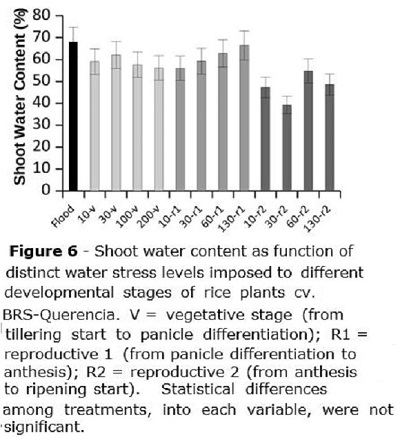
The shoot water content (Figure 6) was equivalent for all treatments in flooded, V and R1 stages, being of about 63%; for R2, mean water content in rice plants was 47%. Inside each developmental group (V,R1,R2), there was no association between rice shoot water content and the stress level to which plants were submitted.
Shoot dry mass was divided into leaf and culm dry mass (Figure 7). Culm dry mass was of about 12 g plant-1 for the flooded check, while it was about 8.52, 7.96 and 11.3 g plant-1, respectively for V, R1 and R2. Water stress imposed at R2 did not affect culm dry mass since rice plants had already reached its maximum growth. For R1, only the higher water stress (130 kPa) resulted in lower dry mass (8.59 g plant-1 as mean of R1-10, R1-30 and R1-60, compared to 6.05 g plant-1 for R1-130) (Figure 7). Leaves dry mass (Figure 7) was about 3.83 g plant-1 for F (flooded), V, and R1, and raised to about 5.82 g plant-1 for treatments imposed in R2.
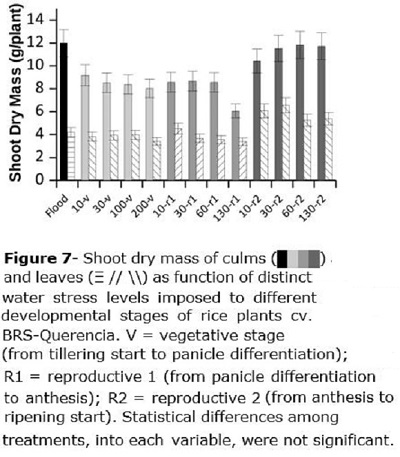
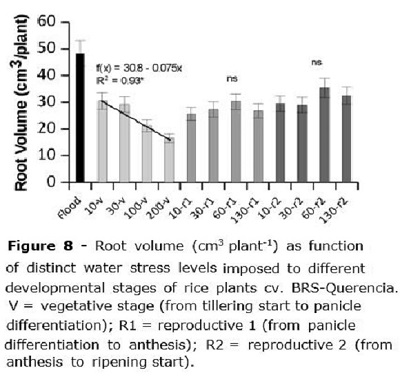
Root volume (Figure 8) was about 48 cm3 plant-1 for the flooded treatment, being superior to any of the irrigated treatments. For treatments applied at the V stage, root volume decreased from 31 to 15 cm3 plant-1 when water stress applied at that stage was increased from 10 to 200 kPa (Figure 8), being the root volume decreased by 0.075 cm3 plant-1 for each additional kPa of water stress. When the stress was applied at R1 or R2 there was no clear effect of water tension on root volume, averaging 27.5 and 31.6 cm3 plant-1, respectively (Figure 8).
DISCUSSION
Plant height was clearly affected when water stress was imposed at V and R1 stages (from tillering start to anthesis) (Figure 3); while plants under stress at V were able to resume growth and completely restore the plant height after the stress was removed, those plants which underwent water stress at R1 did not recover from the stress. These results are according to Gomes and Magalhães Jr. (2004) who reported that rice is able to recover from moderate water stress levels imposed at the vegetative stage, keeping its grain yield potential at harvest. One of the main issues of rice plants with smaller size in production fields, would probably be their reduced ability to compete against weed species (Aldrich, 1984), but on the other side it would also reduce crop lodging risk (Gomes and Magalhães Jr., 2004).
Since tillering (Figure 4) was affected only when the water stress was imposed at V, with no recovery throughout the end of the cycle, it is possible to infer that the small number of tillers per plant under stress at V is minimally compensated by increased leaf area (Figure 5) which was formed after the stress imposed at V was withdrawn. Leaf area is not able to compensate the decrease on the number of grains, consequence of the reduction on the number of tillers. Zain et al. (2014) reported rice tillering mortality and reduced plant height under water stress, but these authors did not assess rice leaf area. Regarding overall plant growth as function of periods of stress, these authors also found that water stress periods lasting for a maximum of five days did not affect rice growth and development.
No water content changes were reported at the present study as function of the distinct water stress levels in soil, at any developmental stage; however, Biswas and Choudhuri (1984) reported water content decrease in rice plants as a function of drought stress. In fact, shoot water stress in rice plants may occur even when there is no drought and water is available, since rice roots have an inherent poor ability to conduct water, causing, in specific conditions, plant water deficit even when roots are into water (Miyamoto et al., 2001). Thus, decreased rice shoot water content may depend mostly on climatic conditions (air humidity and temperature) than on the amount of excessive water present in soil. This represents another evidence that rice may be safely grown under sprinkler irrigation, with no harm to plant development, since irrigation is properly managed.
Regarding the developmental stages in which rice is most susceptible to water stresses, Blum (2011) reports that there is a big amount of experimental data which points for a general rule that plants are usually most severely affected by water stress at the reproductive stage, mainly near flowering (anthesis). When stress is imposed to rice between panicle initiation and anthesis, although a naturally slow rice plant growth is reported in this period, the damage in plant height and tillering is not recovered and plant yield potential may be decreased (Biswas and Choudhuri, 1984).
At the present study, rice plant development was mostly affected by stresses imposed at the vegetative and reproductive stages until flowering. Our findings, however, are according to the reported by Blum (2011) and Biswas and Choudhuri (1984), since rice damage caused by stresses applied to the reproductive stage were not recovered after the stress was withdrawn.
When the water stress was imposed in R1 and R2, plants seemed to direct their reserves of energy to form roots (Figure 8) and try to escape severe water stress. In the V stage, the plant did not have enough reserves and root volume was decreased as the stress level increased. It is usual in Brazilian rice fields to leave rice go through a moderate water stress by the beginning of tillering, as a way to allow increasing in root volume. In the present study, under controlled environment, this was not observed; however, Biswas and Choudhuri (1984) reported that although some water stress did not increase rice development, it reflected in superior yield components at harvest. Our data supplies evidence that this management may not increase root volume or any other growth parameter for currently available early cycle rice varieties.
A relevant result is the much higher root volume at the constantly flooded check compared to the other treatments (Figure 8). Klumb et al. (2012) suggested that the demand of nutrients for rice grown under aerated soils may be higher than the required by flooded rice, due to a series of chemical reactions which could turn fertilizers most available for rice under flooding; thus, additional fertilizer could be needed for rice grown under sprinkler irrigation compared to flooding to keep equivalent plant growth ability and grain yield potential. This, however, could be partially managed by a proper fertilizer formulation (Yang et al., 2012).
Considering the plant height, tillering, leaf area and root volume, it is prudent to report that rice plants growth seem to be little affected by the absence of waterlogging, being possible to be grown under sprinkler irrigation in highlands since water is properly managed to avoid water stress in soil above 30 kPa throughout the cropping cycle.
FINAL CONSIDERATIONS / CONCLUSIONS
Rice plant height is reduced when the water stress is applied either at the vegetative or at the beginning of the reproductive stage (from panicle initiation to anthesis); in the first case, plants are able to later recover from the stress, but this is not true when stress was imposed at the beginning of the reproductive stage.
Tillering was affected only by water stress imposed at the vegetative stage, and plants did not recover after the stress was withdrawn.
Plant water content was not affected by the water stress, but shoot dry mass of culms was reduced when stress was applied between tillering start and anthesis.
Root volume did not increase as a function of water stress imposed at the vegetative stage.
Rice plants growth seem to be little affected by the absence of waterlogging, but there is the need to avoid water stress levels above 30 kPa throughout the cropping cycle.
References
Aldrich, R.J. (1984) - Weed-crop ecology: principles in weed management.: Breton, North Scituate. 465 p. [ Links ]
Biswas, A.K. & Choudhuri, M.A. (1984) - Effect of water stress at different developmental stages of field-grown rice. Biologia Plantarum, vol. 26, n. 4, p. 263‑266. http://dx.doi.org/10.1007/BF02902907 [ Links ]
Blum, A. (2011) - Plant breeding for water-limited environments. Springer, New York. 255 p. [ Links ]
Carlesso, R.; Hernandez, M.G.R.; Righes, A.A. & Jadoski, S.O. (1998) - Índice de área foliar e altura de plantas de arroz submetidas a diferentes práticas de manejo. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 2, n. 3, p. 268‑272. http://dx.doi.org/10.1590/1807-1929/agriambi.v2n3p268-272 [ Links ]
Carli, C.; Steinmetz, S.; Streck, N.A.; Marchesan, E. & Silva, M.R. (2016) - Número de dias e de graus-dia entre a iniciação e a diferenciação da panícula em cultivares de arroz irrigado. Ciência Rural, vol. 46, n. 3, p. 428-433. http://dx.doi.org/10.1590/0103-8478cr20141057 [ Links ]
Correll, D.S. & Correll, H.B. (1975) - Aquatic and Wetland Plants of Southwestern United States. vol. 1. Stanford University Press, Stanford. 1777 p. [ Links ]
Embrapa (2005) - BRS-Querência: precocidade, produtividade e qualidade a serviço da orizicultura Gaúcha. Empresa Brasileira de Pesquisa Agropecuária: Embrapa Clima Temperado, Pelotas. 2 p. < http://ainfo.cnptia.embrapa.br/digital/bitstream/item/44142/1/brs-querencia.pdf> [ Links ]
Gomes, A.S. & Magalhães Jr., A.M. (2004) - Arroz irrigado no sul do Brasil. Embrapa Informação Tecnológica, Brasília. 899 p. [ Links ]
Gurevitch, J.; Scheiner, S.M. & Fox, G.A. (2009) – Ecologia vegetal. Artmed, Porto Alegre. 574 p. [ Links ]
Klumb, E.K.; Scivittaro, W.B.; Silva, P.S. da; Mello, D.C. & Silveira, A.D. (2012) - Absorção de macronutrientes pelo arroz irrigado por aspersão. In: Congresso de Iniciação Científica, 21; Mostra Científica, 4., 2012, Pelotas, RS. Anais. Pelotas, RS: Universidade Federal de Pelotas, v. 1. [ Links ]
Minshall, W.H. (1960) - Water moisture in plants. Canadian Journal of Botany, vol. 38, p. 201-216. [ Links ]
Miyamoto, M.; Steudle, E.; Hirasawa, T. & Lafitte, R. (2001) - Hydraulic conductivity of rice roots. Journal of Experimental Botany, vol. 52, n. 362, p. 1835‑1846. https://doi.org/10.1093/jexbot/52.362.1835 [ Links ]
Parfitt, J.M.B.; Pinto, M.A.B.; Timm, L.C.; Bamberg, A.L.; Silva, D.M. da & Bretanha, G. (2011) - Manejo da irrigação por aspersão e desempenho da cultura do arroz. In: Congresso brasileiro de arroz irrigado, 7, 2011, Balneário Camboriú. Anais... Itajaí: EPAGRI; SOSBAI. p. 461-464. [ Links ]
R Core Team (2016) - R: A language and environment for statistical computing. Vienna: R Foundation. < https://cran.r-project.org> [ Links ]
SOSBAI (2016) – Arroz irrigado: recomendações técnicas da pesquisa para o sul do Brasil. Sociedade Sul-Brasileira do Arroz Irrigado, Pelotas. 197 p. [ Links ]
Yang, X.; Li, Y.; Ren, B.; Ding, L.; Gao, C.; Shen, Q. & Guo, S. (2012) - Drought-Induced Root Aerenchyma Formation Restricts Water Uptake in Rice Seedlings Supplied with Nitrate. Plant Cell Physiology, vol. 53, n. 3, p. 495‑504. https://doi.org/10.1093/pcp/pcs003 [ Links ]
Zain, N.A.M.; Ismail, M.R.; Puteh, A.; Mahmood, M. & Islam, M.R. (2014) - Impact of cyclic water stress on growth, physiological responses and yield of rice (Oryza sativa L.) grown in tropical environment. Ciência Rural, vol. 44, n. 12, p. 2136-2141. http://dx.doi.org/10.1590/0103-8478cr20131154 [ Links ]
Received/recebido: 2016.10.10
Received in revised form/recebido em versão revista: 2017.04.17
Accepted/aceite: 2017.04.18














