INTRODUCTION
The importance of weeds in crop systems is directly linked to their potential economic damage (Oerke, 2006). In the concomitant presence of crops at critical stage of development and weed populations, competition for space, light, water and nutrients intensifies. In this competition, the more rustic species takes advantage, due to their lower physiological requirements, high growth rates and greater tolerance to environmental variations. In addition to these advantages, some species have both vegetative and reproductive propagation, with a high number of seeds, high dissemination capacity and longevity (Inverno et al., 2016; Oliveira et al., 2016).
Weed interference on crops may occur through competition for resources, mainly water, light, nutrients and CO2; through the reduction of the quality of the harvested product, through hosting pests, diseases and nematodes; and through allelopathy (Dalazen et al., 2017). Allelopathy occurs when a donor plant releases into the environment substances that impair or benefit the germination, growth and development of recipient plants (Zeng et al., 2008).
These substances, known as allelochemicals, are produced in the secondary metabolism of plants in different amounts during the year in the organs of plants (leaves, fruits, flowers, stems and roots) and can be released via leaching, exudation and volatilization (Kumari et al., 2017). Allelochemicals comprise 14 categories of substances with different chemical structures, the most common being saponins, coumarins, alkaloids, tannins, terpenoids, quinones, ethylene and flavonoids (Rice, 1974; Tokura et al., 2006).
Allelochemicals may cause delay or inhibition of seed germination, growth stall, root system injury, chlorosis, wilt and plant death (Carvalho et al., 2014; Sartor et al., 2015). Allelopathic mechanisms involve structural alteration of cells, inhibition of cell division and elongation, imbalance of the antioxidant system, increased cell membrane permeability, and alterations in several essential activities, such as plant-growth regulating system, enzymatic functions, respiration, photosynthesis, water and nutrient absorption, and synthesis and metabolism of proteins and nucleic acids (Cheng & Cheng, 2015). These relationships may occur between weed species and crops or among weed species. When they happen among weeds, the consequence is an increase in the distribution and abundance of species with allelopathic potential. When the receiving plant is a crop, the consequence is the reduction of productivity. There are also situations in which cultivated plants have an allelopathic effect on weeds, which favors productivity (Field et al., 2006; Belz, 2007; Zheng et al., 2015).
Sourgrass (Digitaria insularis (L.) Fedde) is native plant species to the Americas, from Argentina in the south to southern USA in the north (GBIF, 2012). D. insularis is one of the main weeds in crop areas in South America (Heap, 2018) and has become relevant due to its resistance to glyphosate in most areas of production (Barroso et al., 2014; López-Ovejero et al., 2017; Oreja et al., 2017). In addition, there are reports of resistance to aryloxyphenoxypropionate (SPF) herbicides in the State of Mato Grosso do Sul, Brazil (Heap, 2018). In that country, this weed is widely distributed in the mid-west, southeast and south regions (López-Ovejero et al., 2017), being present in 8.2 million hectares (Adegas et al., 2017). It is also a weed problem emerging in importance in agricultural fields from the north of Argentina (Oreja et al., 2017). According to Mendonça et al. (2014), the invasive behaviour of sourgrass in cultivated areas is due to its strategy of aggressive regeneration, which is based on seed germination. It is a perennial, herbaceous, tufted and erect species with aggressive characteristics that provide for the maintenance of the species, such as the formation of short and swollen rhizomes (Machado et al., 2008), besides the large amount of seeds produced with easy dispersion by wind (Rua, 2003).
Sourgrass can cause severe reductions in crop productivity. In soybean crop, this weed reduces soybean yield from 23.5% to 44% at densities of 1 to 8 plants/m-2 (Gazziero et al., 2012). Thus, due to the wide distribution, dominance and competitiveness with the crops, it is hypothesized that sourgrass has some allelopathic effect on other plant species. Thus, the objective of this study was to evaluate the allelopathic activity of aqueous extracts of sourgrass leaves and roots on the germination and initial development of weeds and crops.
MATERIAL AND METHODS
The experiment was carried out at the Phytotechnology Laboratory of the State University of Londrina, Brazil. Two factorial experiments (3x2x3) were performed. Factor A was formed by different recipient species, used as bioindicators of the allelopathic effect caused by sourgrass. The first experiment used corn (Zea mays L. cv. ‘AG 9010’, Agroceres®), soybean (Glycine max (L.) Merr. cv. ‘BMX Potência’, Brasmax®) and common bean (Phaseolus vulgaris L. cv. ‘Tangará, IAPAR®). The second experiment used the weeds crabgrass (Digitaria horizontalis Willd.), cockspur grass (Echinochloa crusgalli (L.) Beauv.) and black-jack (Bidens pilosa L.).
Factor B was formed by two origins of aqueous extract: shoot of sourgrass (leaves) and roots. Factor C was composed of three concentrations of the extracts: control (0), 50 and 100%. The sourgrass plants used to produce the extract were collected in the experimental area near the Institution’s laboratory in October 2016 and were in a reproductive stage of development. The 100% extract was obtained by grinding (CUT 4 Metvisa® cutter) 250 g of fresh tissue (shoot or root) in 1 L of distilled water, with subsequent filtering. For the 50% concentration, the pure extract (100%) was diluted in distilled water. For the control treatment, only distilled water was used.
To assess the allelopathic effect in the tested crops, four replicates of 50 seeds were set up on rolls consisting of three sheets of germitest paper, moistened 2.5 times in relation to their dry weight with distilled water (0%) or extract solution (50 or 100%). After set up, the rolls were kept in a Mangelsdorf-type germinator at 25 °C (Brasil, 2009) for six days. The number of normal seedlings was evaluated and represented in percentage.
The experiment with weeds was carried out in transparent Gerbox® plastic boxes (11 x 11 x 3.5 cm), through the disposition of 50 seed per replicate, on a sheet of blotting paper, moistened 2.5 times in relation to its weight with distilled water (0%) (Brasil, 2009) or extract solution (50 or 100%). The experimental units were maintained under BOD with constant light at 25 °C for fourteen days. The evaluations were made based on the number of normal seedlings, expressed as a percentage.
The length of the shoot and root were also evaluated. The methodology was similar to germination for each species, with a differential of ten seeds per replicate, in addition to the Gerbox® maintained at a 45° slope during the incubation period. The measurements were obtained in millimeters with a graduated ruler.
The design was completely randomized, and the data of both experiments were submitted to analysis of variance (ANOVA) and the means were compared by the Tukey test (p <0.05), using the software SISVAR®.
RESULTS AND DISCUSSION
The seed crop germination results (Table 1) indicated that the soybean and corn crops did not have a reduction in percentage of germinated plants with the addition of aqueous extracts of sourgrass. Only the bean crop, in the concentration of 50% of root extract, suffered a significantly reduction in germination, of 12.5% in comparison to the control.
The soybean and bean shoot lengths (hypocotyl) were significantly increased when their seeds were exposed to the extract of the shoot of sourgrass at 100% concentration (Table 1). In the soybean crop, the hypocotyl length increased 2.79 cm (29.3 %) with the 100% concentration in comparison to the control. In the bean crop, in the concentration of 100% of the shoot extract, the increase in growth was even higher, reaching 7.13 cm, which represents a percentage increase of 64.4%. The extract of the shoot of sourgrass did not have any effect on the length of the shoot of the corn plants.
The variation in the concentration of sourgrass root extracts did not affect soybean shoot growth. However, both concentrations (50% and 100%) caused a reduction in shoot growth in the corn and bean crops, of 5.15 cm (50.3%) and 4.07 cm (36.8%) for corn and beans, respectively, at 50% concentration. At the 100% concentration, these values were 3.98 cm (38.9%) and 2.92 cm (26.4%). In general, root extract caused a higher reduction in shoot growth compared to the extract from the shoot of sourgrass.
Table 1 Seed germination and length of soybean, corn and bean seedlings submitted to sourgrass aerial part extract (ExPA) and root system extract (ExSR)
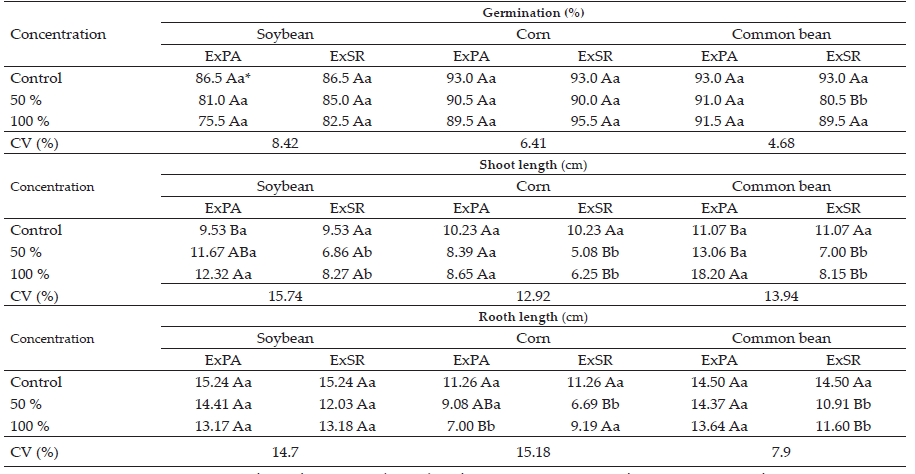
* Means followed by the same letter are not different in Tukey’s test (p <0.05). Capital letters refer to the comparison between concentrations and lowercase letters refer to the comparison of the origin of the extracts.
In the soybean crop, imbibition of the seeds with aqueous extract of sourgrass, regardless of concentration, had no effect on root length (Table 1). In contrast, there were inhibitory effects on root growth of corn and bean crops. The shoot extract only reduced the root system of the corn plants at 100% concentration, with a reduction of 4.26 cm, which represents 37.8% less root length for the plants of this crop. The root extracts caused a reduction in root length both in the corn crop, at 50% concentration, and in the bean crop (in both extracts concentrations). Like in the shoot growth, root extracts caused more inhibitory effects in comparison to shoot extracts.
The absorption of allelochemicals is related to their dilution in the soil solution, which in the case of seeds will be absorbed for imbibition and beginning of the germination process (Nepomuceno et al., 2012). If solution is available in the soil, the way the seed will absorb the compounds varies according to its composition and morphology. There is a tendency for protein seeds, such as beans, to have a higher solution absorption capacity than starchy seeds, and even more than oilseeds, due to membrane permeability and water entry points, which vary according to the species (Faria et al., 2009; Marcos Filho, 2015).
In some allelopathy studies, the common bean crop has already been found to be more sensitive in comparison to other cultivated species. In the case of allelopathy caused by Crotalaria juncea L., common bean germination was more impaired compared to corn, although both were reduced (Araújo et al., 2011). Similarly, a reduction in germination of common bean (farm Ouro Vermelho) was observed when submitted to ethanolic extracts of Bidens pilosa and Cyperus rotundus L. (Monteiro et al., 2014). Rickli et al. (2011), when testing the aqueous extract of neem leaves (Azadirachta indica A. Juss.), verified a reduction in bean root size as the extract dose was increased.
In some situations, it was observed that the sourgrass extracts increased aerial and/or root growth of the recipient species evaluated, and in other species growth was inhibited (Table 1). Faria et al. (2009) analyzed Pinus sp. vegetable extracts, millet (Pennisetum americanum (L.) Leeke) and mucuna (Stizolobium aterrimum Piper & Tracy), on the germination of beans, soybean and corn. The authors observed that the extract of Pinus sp. increased soybean root and hypocotyl, whereas in beans the effect was negative. However, when the beans were submitted to extracts of mucuna and millet, there was an increase in shoot growth.
Thus, the allelochemicals can cause both positive and negative effects on recipient plants. This is because allelochemicals are composed of hundreds of substances, some of which are inhibitory and others that stimulate plant growth. Plant growth regulators, such as gibberellin and ethylene, are also considered allelochemicals (Cheng & Cheng, 2015).
Another important point to consider is the interaction between the allelochemicals, which may be synergistic, additive (null) or antagonistic. Tannins, for example, are known to be antagonists of the action of gibberellin on α-amylase synthesis in barley seeds, which impairs their germination (Jacobson & Corcoran, 1977). Inhibition or induction of initial growth can be explained by the fact that some allelochemicals (caffeic acid, ferulic acid, chlorogenic acid, quercetin, rutin and other polyphenolic compounds) synergize the action of indoleacetic acid (IAA), while others (ρ- hydroxybenzoic and other monophenol compounds) antagonize the action of this growth hormone (Tomaszewski & Thimann, 1966). Thus, the concentration and proportion of certain allelochemicals present in the extract explain why the higher doses are not always the most harmful or stimulant (Souza Filho, 2006).
The allelopathic effect and the allelochemicals present in Digitaria insularis have not been studied and identified. Within this genus, the allelopathic activity of Digitaria sanguinalis on soybean, wheat and corn was studied, with a significant reduction in plant growth (Zhou et al., 2013). In these species, the allelopathic effect was attributed to the presence of three compounds present in the roots of the weed: veratric acid, maltol and loliolide. Similarly, Oryza sativa L. allelopathy on cockspur grass is also attributed to compounds present in the root system of the crop, momilactone B, flavone and cyclohexanone (Kong et al., 2002).
Corroborating with these observations, the present study found that the common bean crop was sensitive to the root extract of the sourgrass, both for germination and the growth of aerial parts and root (Table 1). Thus, in practice, it would not be advisable to sow, especially, common bean in an area with high infestation of sourgrass, due to the possible allelopathic effects.
However, it is important to note that in the soil there are several interactions between the allelochemicals and abiotic and biotic factors that can interfere with this negative effect, so that can not be observed (Tharayil et al., 2008; Zhou et al., 2013). Therefore, studies that identify sourgrass allelochemical compounds as well as their behavior in the soil are necessary to confirm the participation of allelopathy on the interference of sourgrass on crops and weeds.
The weeds crabgrass and black-jack were sensitive to extracts of the shoot of sourgrass at both concentrations tested (Table 2). For crabgrass, germination reduced from 62% in the control to 35.5% and 44.5% in the concentrations of 50% and 100% of the extract. For black-jack, germination reduced from 67% in the control to 51% and 39.5% in the two extracts concentrations evaluated. In this case, the 100% concentration was significantly more inhibitory than the 50%.
Regarding the extract from sourgrass roots, black-jack germination was inhibited at both concentrations, from 67% in the control treatment to 45.5% and 48.5% in the concentrations of 50% and 100%, respectively. For cockspur grass, inhibition of germination was only observed at 100% concentration of the root extract, of 22.4% in relation to the control. For crabgrass, at the concentration of 100% of root extract, induction was observed in the germination, demonstrating an inductive effect.
The length of the shoot of the weed species assessed (Table 2) was influenced both by the sourgrass shoot extract and the root extract. The shoot extract reduced the root growth of all species assessed at 100% concentration. The biggest difference in root growth was observed for black-jack, in which the root extract at 100% caused a reduction of 59.1% in shoot length, going from 4.08 cm in the control to 1.67 cm. The concentration of 50% also reduced the shoot in 40.9%, or 2.41 cm in relation to the control. For the crabgrass and the cockspur grass, the reduction in growth of the shoot was of 31.0% and 54.3%, respectively.
The sourgrass root extract reduced the shoot growth of black-jack at both concentrations, with a reduction of 29.2% (-1.19 cm) and 24.5 % (-1.00 cm) for the 50% and 100% concentration extracts, respectively. In the cockspur grass, the growth reduction was significantly different than the control only at 100% concentration, with a reduction of 34.7% (-1.63 cm).
Root weed growth varied in response to the use of sourgrass extracts, including inhibitory and stimulatory responses. (Table 2). The shoot extract inhibited root growth in crabgrass and black-jack in 70.7% (-1.57 cm) and 74.9% (-1.76 cm), respectively, at the 50% concentration. At the concentration of 100%, the sourgrass shoot extract caused a reduction in the shoot growth only in black-jack, of 84.3% (-1.98 cm). Thus, there appears to be a large allelopathic effect of aqueous extracts of sourgrass shoots on early growth of crabgrass and black monkey shoots. On the other hand, in cockspur, no effect was observed in response to shoot extract.
Table 2 Germination and seedling length of crabgrass (Digitaria horizontalis), black-jack (Bidens pilosa) and cockspur (Echinochloa crus-galli) submitted to the sourgrass aerial part extract (ExPA) and the root system extract (ExSR)
| Concentration | Germination (%) | |||||
|---|---|---|---|---|---|---|
| Crabgrass | Black-jack | Cockspur | ||||
| ExPA | ExSR | ExPA | ExSR | ExPA | ExSR | |
| Control | 62.0 Aa | 62.0 Ba | 67.0 Aa | 67.0 Aa | 76.0 Aa | 76.0 ABa |
| 50% | 35.5 Ba | 64.0 Bb | 51.0 Ba | 45.5 Ba | 74.5 Aa | 79.0 Aa |
| 100% | 44.5 Ba | 82.5 Ab | 39.5 Cb | 48.5 Ba | 80.0 Aa | 59.0 Bb |
| CV (%) | 16.02 | 10.25 | 13.78 | |||
| Concentration | Shoot length (cm) | |||||
| Crabgrass | Black-jack | Cockspur | ||||
| ExPA | ExSR | ExPA | ExSR | ExPA | ExSR | |
| Control | 1.92 Aa | 1.92 ABa | 4.08 Aa | 4.08 Aa | 4.70 Aa | 4.70 Aa |
| 50% | 1.79 Aa | 1.66 ABa | 2.65 Ba | 2.89 Ba | 3.86 Aa | 3.72 ABa |
| 100% | 0.59 Bb | 2.04 Aa | 1.67 Cb | 3.08 Ba | 2.55 Ba | 3.07 Ba |
| CV (%) | 12.48 | 9.58 | 15.19 | |||
| Concentration | Root length (cm) | |||||
| Crabgrass | Black-jack | Cockspur | ||||
| ExPA | ExSR | ExPA | ExSR | ExPA | ExSR | |
| Control | 2.40 Aa | 2.40 Ba | 2.35 Aa | 2.35 Ba | 4.30 Aa | 4.30 Ba |
| 50% | 0.83 Bb | 5.20 Aa | 0.59 Bb | 4.95 Aa | 3.92 Ab | 7.54 Aa |
| 100% | 1.73 Ab | 5.23 Aa | 0.37 Bb | 4.11 Aa | 4.34 Ab | 7.06 Aa |
| CV (%) | 15.65 | 22.45 | 12.4 | |||
* Means followed by the same letter are not different in Tukey’s test (p <0.05). Capital letters refer to the comparison between concentrations and lowercase letters refer to the comparison of the origin of the extracts.
The root length of all weed species evaluated was longer, regardless of concentration, when the seeds were soaked in sourgrass root extract, showing a pronounced inducing effect. In crabgrass, the roots exposed to the extract had a length of approximately 116.7% (+ 2.80 cm) higher than the control. In black-jack, the increase in root length was 92.7% (+2.18 cm). Finally, cockspur roots were 69.7% (+3.00 cm) longer than the control treatment plants.
Some studies have already demonstrated the allelopathic effect of some plant species on weeds used as target species in the present study. The negative effects of Plectranthus barbatus Andrews and rapeseed (Brassica napus L.) on the germination and vigor of B. pilosa have been described (Rizzardi et al., 2008; Azambuja et al., 2010). The authors attributed the allelopathic effect of rapeseed to the decomposition of glucosinolates in isothiocyanates and thiocyanates during germination, which in high doses react with the enzymes necessary for the germination process. Similarly, the aqueous extract of Lupinus angustifolius L. interfered on the vigor of B. pilosa, negatively reducing the root and shoot length (Gomes et al., 2013).
In cockspur, another target species of this study, it has been observed that the leaf extracts of Annona glabra L. (Matsumoto et al., 2010) and root extracts of Sapindus saponaria L. (Grisi et al., 2013) act negatively on the germination and the initial development of this weed. One striking and successful example of allelopathy on cockspur is the genetic improvement of rice crops (O. sativa) with allelopathic potential (up to 65% suppression) on this, which is one of the main weeds of the crop (Belz, 2007). Wheat and rice crops have been reported to suppress D. insularis because of its allelopathic effect, impairing germination and vigor (Li et al., 2005; Brunes et al., 2016).
Therefore, the allelopathic effect of sourgrass may be related to its great competitiveness for crop resources, as well as to its dominant character in areas after its introduction. Thus, integrated management measures, which eliminate or reduce the presence of this weed in the crops, become even more necessary.
CONCLUSIONS
The common bean crop is sensitive to the allelopathic effect of aqueous extracts of sourgrass roots. Both germination and initial growth are hampered. The initial growth of corn crop is also damaged, mainly in response to root extract, although no reduction in germination was observed. There are no negative effects on soybeans. In relation to weeds, black-jack presents greater sensitivity to the allelopathic effect of the shoot sourgrass extract at the highest concentration tested. The other weed species studied also present reduction in at least one of the evaluated variables; crabgrass is the less sensitive one.














