INTRODUCTION
Solanum betaceum Cav. is a species native to the Andean region of South America (Bohs, 1991; Prohens and Nuez, 2001), commonly called tamarillo, tree tomato or “tomate de la paz” (Boris, 1927).
Tamarillo represents an alternative crop for agricultural diversification and it has aroused remarkable interest in New Zealand that is world’s largest producer. The fruits present high nutricional values, with relatively high levels of proteins, vitamins, minerals (McCane and Widdowson, 1992) and components with important antioxidant, therapeutic and preventive properties (Kou et al., 2009; Villegas-Ruiz et al., 2012; Noor Atiqah et al., 2014). It has also good developing prospects in some Mediterranean countries (Prohens and Nuez, 2001; Acosta-Quezada et al., 2011). The plant's morphology being very homogeneous, but there is a considerable diversity of tamarillos that differ in color, shape and flavor. In the bibliography there are those who distinguish them as being different cultivars (Prohens and Nuez, 2001; Bakshi et al., 2016) and there are those who say they are different varieties (Boyes and Strübi, 1997; Pantoja et al., 2009; Vasco et al., 2009).
The determination of germinative characteristics of tamarillo seeds are essential to maximize its utility and provide relevant information about the viability of ex situ seeds conserved.
Optimum germination conditions for this species are not yet well known (Torres-González, 2019), and studies on biology of tamarillo seeds in Portugal are scarce.
However, it is known that germination in Solanaceae species is frequently light sensitive, there may be greater or lesser light dependence during seed germination (Torres-González, 2019). Also, it is known that water plays a fundamental role in the germination process by activating seed metabolism (Villela, 1998), and that temperature influences the intensity and speed of this process (Bewley and Black, 1994).
It is essential that stored seeds have the capacity to germinate and to be used whenever necessary (Cárdenas et al., 2004), and that is why it is important to carry out viability controls periodically (Draper et al., 2004). Considering that, this study arises with the objective of analyzing the characteristics related to the germination of tamarillo seeds, as a complement to the study on the ex situ conservation seeds, for knowledge of the germinative characteristics of these fresh seeds that can serve as a comparison to future tests of conserved seeds viability.
MATERIAL AND METHODS
The fruits used in the study were harvested from the trees of the Botanical Garden of the Coimbra University, Portugal. Subsequently, the seed cleaning operations took place in the Plant Biotechnology Laboratory of the Department of Life Sciences - College of Sciences and Technology, University of Coimbra.
Were used red (from 5 trees desiganted R1, R2, R3, R4 and R5) and orange (designated O) ripe tamarillo fruits. The seeds were extracted from the tamarillos and properly cleaned to eliminate pulp remnants from the fruits. Then, samples of 20 orange tamarillo seeds and 100 red tamarillo seeds were constituted - 20 seeds of Ri accessions were placed to germinate.
The germination test occurred immediately after the seeds were removed from the fruits. It was carried out in Petri dishes with cotton and filter paper moistened with distilled water until saturation (Silva, 2012; Maciel et al., 2018). The cotton and filter paper were rehydrated whenever necessary.
Two Petri dishes were prepared for each of the previous six samples of 20 seeds, and 10 seeds of the same fruit strain were placed in each dishe (2 x 10 of O; 2 x 10 of Ri). The seeds were set to germinated in a chamber at 25ºC at total darkness or with a photoperiod of 16 hours of light and 8 hours of darkness (Torres-González, 2019), with a white light and 15-20 µmol m-2 s-1 of intensity.
After 60 days, Petri dishes with seeds that had not yet germinated, were removed from the chambers with a controlled environment and placed in laboratory natural conditions, near by a window.
Seed germination was defined by the radicle emergence (Villamil and Laborde, 2001; Silva, 2012).
The germinated seeds were subsequently removed from the Petri dishes, and those that remained were reorganized in space in order to standardize the physical conditions of germination (Silva, 2012).
The germinated and non-germinated seeds were counted, and the following were calculated:
i) Maximum Germination Rate = germinated seeds number total number of seeds−empty seeds number x100
(Bacchetta et al., 2008; Silva, 2012).
ii) Germination Speed - time required to obtain 50% of the seeds germination capacity (Côme, 1970; Silva, 2012), expressed in days.
iii) Germination Period Duration - time between the moment when 10% and 90% of maximum germination is reached (Baskin and Baskin, 2001; Silva, 2012), expressed in days.
iv) Total Germination Time - number of days required for all seeds to germinate.
For the red accessions tamarillo seeds was performed a statistical treatment of the results through the Tukey test, for a significance level of 0.05, using the IBM SPSS Statistics 26 software.
For linear regressions established between some variables was also considering a significance level of 0.05, in the regression parameters analysis.
RESULTS AND DISCUSSION
It was found that there were differences in the Solanum betaceum Cav. promotion of germination, as was also verified by Torres-González (2019). Germination varied among seed samples depending on the germination conditions to which they were submitted (photoperiod or abcense of light).
Shortly after sowing, the orange accessions seeds began to germinate, whether they were in the absence of light or those that were subjected to the photoperiod, but there was greater difficulty in red accessions tamarillo seeds to germinate.
For the orange accessions tamarillo seeds, the Germination Speed (GS) was 16 days for those who were under photoperiod and 17 days for those who were in absence of light; and the Maximum Germination Rate (MGR) was 100% for both cases (Table 1). The Germination Period Duration (GPD) was 14 days for those who were under photoperiod and 56 days for those who were in the absence of light (Table 1). It took 69 and 27 days for all seeds that were in absence of light and under photoperiod, respectively, to germinate (Table 1). All of which germinated, effectively, in the absence of light or under photoperiod (environmental conditioning) (Figure 1), although it is notorious that germination was favored by the presence of light, also as found by Neto et al. (2015).
Table 1 Expression of the results of seed germination of Solanum betaceum Cav. to the percentage of maximum germination rate (MGR), days of germination speed (GS), days of germination period duration (GPD) and days of total germination time (TGT)
| Photoperiod/Lab. conditions | Absence of light/Lab. conditions | |||||||
|---|---|---|---|---|---|---|---|---|
| Accessions | MGR | GS | GPD | TGT | MGR | GS | GPD | TGT |
| (%) | (days) | (days) | (days) | (%) | (days) | (days) | (days) | |
| R1 | 100 | 80 | 5 | 97 | 100 | 94 | 7 | 104 |
| R2 | 100 | 73 | 56 | 76 | 100 | 87 | 6 | 107 |
| R3 | 100 | 63 | 43 | 65 | 100 | 79 | 5 | 82 |
| R4 | 100 | 74 | 51 | 88 | 100 | 88 | 6 | 98 |
| R5 | 100 | 80 | 11 | 97 | 100 | 104 | 17 | 114 |
| O | 100 | 16 | 14 | 27 | 100 | 17 | 56 | 69 |
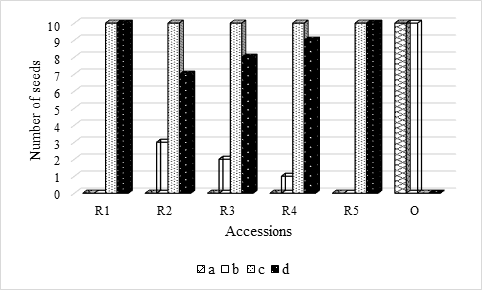
Figure 1 Solanum betaceum Cav. number of seeds (n = 20) that germinated: a) in absence of light; b) under the photoperiod 16h light/8h darkness; c) in laboratory conditions from the absence of light; d) in laboratory conditions from the photoperiod 16h light/8h darkness.
The red accessions tamarillo seeds were having little success in germination, and after 2 months to sowing, in a controlled environment, they were placed in natural and less conditioned environmental conditions, simply near by a window. In general, a maximum germination period is established (e.g. Maciel et al., 2018), after which, seeds that have not germinated are subjected to tests to assess their viability (e.g. tetrazolium stain test [Costa and Santos, 2009] or cut-tests [Silva, 2012]). This was not done, because the objective was to effectively study the germinative characteristics of these seeds, and to have some reference for this species, as their optimal germination conditions are not yet well known (Torres-González, 2019).
According to Acosta-Quezada et al. (2011), the red and orange tamarillos are the most distinct in morphological terms. Something that will be due to a genetic basis that determines it and possibly also has an influence on the germinative characteristics of these seeds. It would have been interesting to have more orange accessions seeds samples (and other accessions) to see if there is intraspecific variability among Solanum betaceum Cav. seeds germination.
There were also differences in germination between seed samples of the red accessions tamarillos. Something that was also observed by Andreoli and Khan (1999), and Godefroid et al. (2010), who state that consistent results were not always obtained within the same species, with varying individual germination results; and Neto et al. (2015) state that the tamarillo has germination unevenness when multiplied by seeds.
By observing Table 1, it can be seen that the Total Germination Time (TGT) was higher in the seeds that had initially been in absence of light, which is an indication that the seeds of the red tamarillos are sensitive to the presence of light to germinate (seeds dormancy breaking - Neto et al., 2015; Maciel et al., 2018; Torres-González, 2019). These results are in agreement with those presented in Figure 1, which show that no seeds of the red accessions germinated in the absence of light, and they are corroborated by Neto et al. (2015) who observed that the germination of tamarillo seeds tends to be favored by the presence of light.
However, after being placed in laboratory conditions, the seeds that were initially in absence of light, on average, had a lower GPD compared to those that were under photoperiod. In particular, only R1 and R5 accessions opposed this trend with 2 and 6 days difference, respectively.
Also the GS was, on average, higher in the seeds that were initially under photoperiod (Table 1), that is, the number of days until 50% of the seeds germinated was lower in those that received light right from the beginning of the imbibition/germination process, which again reinforces the idea that the presence of light is essential for the red strain tamarillo seeds germination. This behavior disagrees with the results presented by Maciel et al. (2018) who state that the germination speed of tamarillo seeds was similar in the presence and absence of light.
Nevertheless, these results are in line with those obtained by Neto et al. (2015), who found that seeds obtained from fresh fruits have a higher percentage of germination when exposed to light. These authors, also report that although there was a prevalence of germinative superiority in the seeds with presence of light, it was not possible to determine whether the tamarillo seeds are photoblastic positive, once in the absence of light there was also germination, as happened in the present study (for the orange accessions).
In statistical terms (data not showed - for the red accessions), GS and GPD are significantly higher in seeds that have undergone the photoperiod regime, compared to those that were initially in absence of light. However, there were no significant differences in TGT between these two regimes; neither in the number of days that the seeds in absence of light or under photoperiod, were in laboratory conditions until all germinated.
This means that germination conditions play a fundamental role in the germination of red tamarillo seeds, as regardless of GS and GPD, which represent the time required to obtain 50% of the germination capacity of the seeds and the time that mediates the moment in that 10% and 90% of maximum germination is reached, respectively, there are no significant differences in the number of days that the seeds were in laboratory conditions until all germinated (quantitatively represented by Figure 2). This statement is further supported by Figure 1, where the effect of environmental conditions (at least in relation to what was tested) on seed germination is notorious.
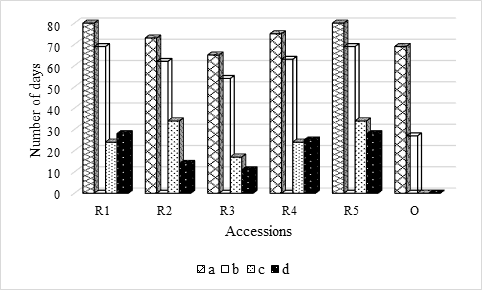
Figure 2 Solanum betaceum Cav. number of days that seeds were set up: a) in absence of light; b) under photoperiod16h light/8h darkness; c) from absence of light have been in laboratory conditions until they all germinated; d) from photoperiod 16h light/8h darkness, have been in laboratory conditions until they all germinated.
According to Cochrane (2004), there is still an insufficient understanding of the complex dormancy mechanisms that prevent seed germination under standard laboratory conditions of humidity, temperature and light, which makes it difficult to evaluate the response of the seeds to germination conditions and also to long-term storage conditions.
Neto et al. (2015) and Torres-González (2019), claim that tamarillo seeds have physiological dormancy - which is that in which the presence of inhibitory factors or the absence of promoting factors, prevent germination from occurring (Baskin and Baskin, 2001) - which can be successfully broken by exposing the seeds to light.
In addition to the presence of light, Maciel et al. (2018) and Torres-González (2019), also mentions that the best temperature to promote the tamarillo seeds germination was an alternating temperature, to the detriment of a constant temperature. That said, it is believed that the germination of the red tamarillo seeds, in this study, will have been favored, not only by the presence of light (relative to those in absence oh light), but also by the temperature change from 25ºC (environmental conditioning) to an average temperature of 18.8ºC in the laboratory.
The differences in the number of days that the seeds of the red accessions were in abcense of light or under photoperiod, are due to the fact that the tamarillos were harvested on different dates due to the degree of ripeness, having occurred between November 5 and 20 (2019).
Given that there were seeds germinated under photoperiod (red accessions), it was decided to establish a regression analysis between the number of days that seeds were under environmental conditioning or in laboratory conditions, and the number of seeds germinated in each situation. The results revealed the existence of a strong correlation (R2= 0.82) and significant (p = 0.04), between the number of days that the seeds were under laboratory conditions and the number of germinated seeds. However, between the number of days under photoperiod and the number of seeds that germinated under these conditions, there was only a moderate (R2 = 0.52) and non-significant (p = 0.17) correlation. For the latter situation (under photoperiod), the slope of the regression line was negative, thus indicating that the germinative capacity of the seeds will be reduced the longer they remain under less favorable conditions for germination.
These results confirm, once again, what was said before regarding the environmental influence on the germination of red accessions tamarillo seeds. Therefore, germination requirements can differ significantly between accessions (Andreoli and Khan, 1999; Godefroid et al., 2010; Neto et al., 2015), and the reasons for differences in germination capacity within the same species are varied, and may include a genetic basis (Walters et al., 2005), differences in sensitivity to environmental conditions and dormancy degree (Baskin and Baskin, 2001). And as verified by Godefroid et al. (2010), that the use of the same protocol produced robust results, while different protocols provided very different germination and viability percentages, the change in the methodology used in this test in relation to environmental characteristics, had different effects on the germination of the red strain seeds.
Thus, it is necessary to try to exploit to the maximum the critical factors that affect the seed germination and to try to establish a standard by which to evaluate their quality/viability. This standard, which should correspond to germination tests under ideal conditions (Godefroid et al., 2010).
CONCLUSIONS
Considering the results obtained, it is concluded that: i) there are differences in the promotion of germination in Solanum betaceum Cav.; ii) the germination conditions play a fundamental role in the tamarillo seeds germination; iii) germination of tamarillo seeds, tends to be favored by the presence of light.
It also highlighted, the importance of carrying out germination tests on fresh seeds/post-harvest, in order to know the germinative characteristics of the seeds and to have a comparison term for future tests of conserved seeds viability.
In the future, this study would be worthy of a broader research to better understand the tamarillo seeds biology and contribute to the establishment of specific and accessions analysis protocols.














