INTRODUCTION
Plant parasitic nematodes (PPNs) are small roundworms that greatly affect crops in modern agriculture. They belong to the phylum Nematoda and are characterized by the presence of the stylet, a piercing mouthpart that aids in plant parasitism. Root-knot nematodes (RKN), of the genus Meloidogyne, and cyst nematodes (CNs), of the genus Globodera and Heterodera, are the most economically important soil-dwelling PPNs, responsible for heavy losses to farmers. Recently, the damaging effects of PPN diseases have increased dramatically, due to globalization and monoculture mass production, requiring more effective means of pest management.
Pest management is usually performed through non-chemical means, relying on natural host resistance or on cultural controls, and chemically, using nematicides. Nematicide screening is mainly performed using in vitro direct contact assays or in vivo screening. However, direct contact assays are unable to reflect phytotoxicity to the host or compound biotransformation and metabolization, while in vivo screening is an extremely lengthy and unpractical process, beset by environmental and genetic variations.
An innovative screening procedure was developed based on in vitro cultures of transgenic roots with soil PPNs (Faria et al., 2014), and validated as being able to evaluate nematicidal activity and toxicity to the host, simultaneously, in an easily accessible biotechnological system (Faria et al., 2015). In vitro co-cultures are refined screening systems, kept under controlled nutritional and environmental conditions, with defined and easily manipulated biological parameters (Faria et al., 2016). Furthermore, co-cultures need fewer resources, in terms of space and time, offering additional advantages to conventional in vivo systems for large-scale screening of nematicidal compounds.
In the present work, Solanum lycopersicum and S. tuberosum transgenic roots were established as in vitro culture hosts for Meloidogyne ethiopica and Globodera pallida. These plant - parasite biotechnological systems will be used to obtain aseptic phytoparasitic nematodes in large amounts and to futurely test biopesticide compounds.
MATERIAL AND METHODS
Tubers of S. tuberosum cv. Agria and fruits of S. lycopersicum cv. Cerasiforme were obtained locally. Samples were washed with commercial bleach 20% (v/v), for 10 min, and surface sterilized by immersion in ethanol 96% (v/v) for 10 min. Under asepsis, potatoes and tomatoes were rinsed 3 times in ultrapure sterile water, the outer 2 cm portion was removed, and the central piece divided into segments with approximatelly 0.5 cm of thickness.
Transgenic roots were induced by inoculating the aseptic potato or tomato sections with Rhizobium rhizogenes ARqual strain, according to the previously described protocol (Faria et al., 2014). Transgenic roots were then maintained in semi-solid Schenk and Hildebrandt (SH) medium supplemented with 30 g L-1 sucrose and 8 g L-1 agar at pH = 5.6. Plates were kept at 24±1ºC, in darkness, under regular subculturing.
Co-cultures were established according to Faria et al. (2014). For M. ethiopica, egg masses were handpicked from infected tomato plants from the in vivo nematode collection of INIAV’s Nematology Lab (Rusinque et al., 2022). The eggs were extracted with 0.52% (v/v) sodium hypochlorite (NaOCl) solution and the debris removed with a 47% (w/v) sucrose solution. Afterwards, second stage juveniles (J2) were hatched from the eggs in moist chambers and surface sterilized in 70% ethanol for 2 min, centrifuged for 2 min at 500 g, and finally rinsed in sterile water (4 times) to eliminate traces of ethanol. For G. pallida, cysts were provided by the cyst nematode collection of Nematology Lab of INIAV. Cysts were extracted from the soil samples using the Fenwick can method, eggs and J2 were recovered from cysts and surface sterilized as described above. Approximately 50 M. ethiopica juveniles or G. pallida eggs were added to S. lycopersicum or S. tuberosum transgenic roots, respectively, and kept for 2 months for infection and female maturation.
Results and discussion
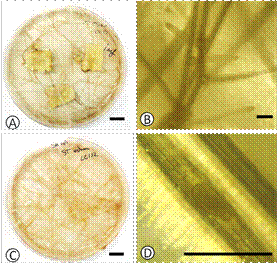
For in vitro infected tomato sections, transgenic roots began emerging from etiolated seedling hypocotyls, from the seeds that immediately germinated of the tomato sections. After a month, transgenic roots were already evident (Figure 1A). These roots were excised and periodically subcultured (Figure 1B and C). The aseptic M. ethiopica J2 added were identified in the vicinity of tomato roots and after approximately 2 weeks the first indications of root galling were detected (Figure 1D).
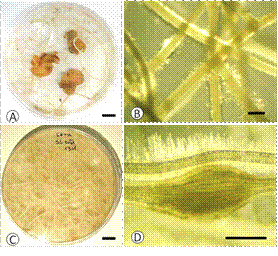
Figure 1 (A) Visual aspect of transgenic roots developing from aseptic tomato sections, (B) microscopic aspect of a tomato transgenic root, (C) transgenic roots culture in semi-solid SH culture medium and (D) a root gall encasing a maturing Meloidogyne ethiopica female. Bar = 1 cm for A and C; and 1 mm for B and D.
For potato sections, transgenic roots began emerging from small cell clusters formed in the surface of the section. Within two months root primordia were discernible, and in the following weeks transgenic roots began growing throughout the culture medium (Figure 2A, B and C). The added aseptic G. pallida eggs hatched within the first week and J2 were observed trusthing their stylet at the root epidermis. After approximately one month the first maturing females were identified on wounds in the root (Figure 2D).
CONCLUSIONS
Transgenic roots offer many advantages for plant-parasitic nematode research. At the Plant Nematology Lab of INIAV, they will be used to maintain a reference collection of PPN races, to obtain high quantities of aseptic PPNs for nematicide screening and as novel biotechnological tools to enhance procedures of biopesticides screening.