INTRODUCTION
Asthma is one of the most common chronic diseases worldwide. Incidences amongst general population ranging from 1 to 18% have been described.
Severe asthma accounts for 4 to 10% of all asthma cases. It is defined as uncontrolled disease despite adherence with maximal optimized therapy and treatment of contributory factors and comorbidities, that worsens when high dose treatment is decreased or that can only be controlled with therapies that have intolerable side effects1,2. It is associated with substantial morbidity and mortality3. Its burden is incredibly high, either considering life quality or healthcare related costs3. In Portugal, asthma in adults accounts for over 2% of total healthcare costs, with uncontrolled disease costs being more than double of controlled disease4.
Individualized therapy based on asthma endotype is being pursued and is currently recognized as a more efficacious alternative for patients with symptoms refractory to guideline‑based therapies1.
Monoclonal antibodies that target interleukin (IL)‑5 have shown favorable results in clinical trials, with evidence of reductions in asthma exacerbations and other important clinical outcomes1.
CASE DESCRIPTION
We present the case of a 56‑year‑old woman with a 40‑year long history of asthma and allergic rhinitis. She reported specific allergen immunotherapy during childhood (no further information provided), with poor results. No smoking habits, significant exposure or other medical or surgical relevant diseases.
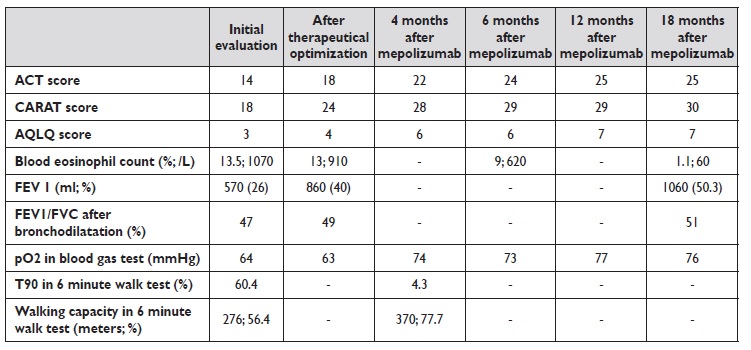
The patient was sent to our Asthma outpatient clinic by her general physician due to uncontrolled respiratory symptoms, with an asthma control test (ACT) score of 14 and control of allergic rhinitis and asthma test (CARAT) score of 18. At this point, she was already medicated with low dose inhaled corticosteroid, long acting beta agonist, long acting antimuscarinic, nasal corticosteroid and anti‑histamine.
She was still presenting three to four exacerbations per year, all of which needing systemic corticosteroid to achieve symptom control. She identified the disease as a major factor of poor quality of life, recognizing a great impact on her everyday working and leisure activities.
Initial blood tests revealed high eosinophil count (13,5%; 1070/L) and total imunoglobulin E of 81.8 UI/mL. Pulmonary function tests (PFT) at rest displayed a severe obstructive defect with FEV1 of 570 ml (26%) and FEV1/FVC index after bronchodilation of 47% (fractional exhaled nitric oxide is not available at this hospital). Arterial blood gas test at rest showed a low pO2 (64 mmHg), with normal pCO2 (41 mmHg). The only pathological finding on chest computed tomography (CT) with contrast was generalized bronchial thickening. Six‑minute walk test was consistent with desaturation with exercise (T90 of 60.4% with minimal value of peripheral oxygen saturation of 86%) and short walking capacity (276 meters, 56.4% of predicted value).
Bronchoscopy was innocent, as well as the cytological, bacterial and mycobacterial exams of bronchial secretions and bronchoalveolar lavage, which displayed macrophages and inflammatory cells, without neoplasic findings. Skin prick tests performed in our hospital were negative, which could explain the poor results to specific allergen immunotherapy during childhood. Echocardiography was normal.
Therapy was progressively increased, with establishment of high dose inhaled corticosteroid and leukotriene receptor antagonist. Comorbidities known to worsen asthma, such as gastroesophageal reflux disease, were considered and properly addressed. Obesity and obstructive sleep apnea syndrome were ruled out (body mass index 26 kg/m2). Ambulatory oxygen therapy to use during exercise was started.
During the first two years of follow up, the patient was evaluated every three to four months. The fact that she always presented with uncontrolled symptoms (ACT scores ranging from 12 to 18, CARAT scores ranging from 18 to 24 and AQLQ scores of 3 to 4) and the sustained need of oral corticosteroids on top of the above implemented strategies, along with the exclusion of differential diagnosis such as Churg‑Strauss syndrome and allergic bronchopulmonary aspergillosis, led to the diagnosis of severe eosinophilic asthma.
Subsequent blood tests consistently showed elevated blood eosinophil count and PFT also had similar findings to the ones previously described. Six‑minute walking test showed that oxygen therapy had improved desaturation but no change in walking distance was noticed.
The patient underwent cardiopulmonary exercise testing, with abnormal findings due to obstructive disease. She was enrolled in an exercise program which went on for three months, with a slight positive impact on dyspnea and on walking distance in the six‑minute walking test (325 meters, 67.2% of predicted value). Anti‑IL‑5 therapy with mepolizumab was initiated.
After the first dosis, immediate improvement was perceived regarding symptom control (ACT score 22, CARAT score 28 and AQLQ score 6). Within four months, ambulatory oxygen therapy was ceased, with arterial blood gas test at rest displaying a better than ever value of pO2 (74 mmHg) and six‑minute walking test with no desaturation and a walking distance of 370 m (77.7% of predicted value). Regarding PFT, FEV1 was 1060 ml (50.3%) and FEV1/FVC index was 51% after 18 months. Blood eosinophil count decreased with time, with values of 9% (620/L) at 6 months evaluation and 1% (60/L) at 18 months.
Table 1 summarizes clinical, laboratory and functional data. For the time being, she has completed 18 months of therapy with mepolizumab. ACT scores are invariably over 23, no admissions to the emergency department or the wards were registered and there was no demand of systemic corticosteroid therapy. Moreover, inhaled corticosteroid dose was reduced and long acting antimuscarinic discontinued.
ACT - asthma control test; CARAT - control of allergic rhinitis and asthma test; AQLQ - asthma quality of life questionnaire
The patient has now no limitations in her everyday life and acknowledges biologic therapy as a life changing therapeutic option.
DISCUSSION
From a functional point of view, asthmatic patients may develop chronic irreversible airflow limitation. It develops as a physiological consequence of chronic airway inflammation and remodeling, which includes large airway epithelial basement membrane thickening, airway Wall oedema and airway smooth muscle hyperplasia and hypertrophia5.
There is little radiological evidence of lung destruction in non smokers asthmatics. The long term consequences of these findings are not fully known5.
Adult population with asthma may present with other comorbidities, such as cardiac failure, chronic obstructive pulmonary disorder and obstructive sleep apnea syndrome, which can contribute to impaired gas exchanges. Furthermore, increasing evidence suggests that asthma may be associated with greater risks of pulmonar embolism6 which could obviously contribute to respiratory failure. Chronic respiratory failure exclusively due to asthma is an uncommon event, as documented in a retrospective study of a population of patients under long term oxygen therapy5.
In patients with severe asthma, both common clinical features and rarer consequences such as respiratory failure are difficult to address and control. Biological therapy has shown promising results in many studies over the past few years and its implementation has undoubtedly enhance the approach to patients with severe asthma.
The fact that it constitutes a targeted therapy makes it more effective, with better and faster results.
Overproduction of IL‑5 was first linked to asthma in the 1990s. To this date, many studies have evidenced a central role for IL‑5 and eosinophilic airway disease, hence the development of IL‑5 targeted therapy1. Mepolizumab and reslizumab are humanized monoclonal antibodies that bind IL‑5 and benralizumab is a humanized monoclonal antibody that binds to the IL‑5 receptor alpha subunit7.
Mepolizumab is the most studied anti‑IL‑5 in the treatment of severe asthma and was the first agent in its class to be tested in a clinical trial8. Evidence has emerged on several benefits with mepolizumab therapy in patients with severe eosinophilic asthma. It has proved to reduce sputum and blood eosinophil count8,9, improve symptom control and lung function8 and decrease asthma exacerbations3,8
and systemic corticosteroid use8,10.
All in all, this is a case of a patient with uncontrolled asthma symptoms that negatively affected her life quality. Moreover, we believe that severe asthma itself led to chronic respiratory failure that met criteria to ambulatory oxygen prescription. The onset of mepolizumab was of the upmost importance, as illustrated by the positive outcomes above described.
CONCLUSION
This case highlights the relevant role of biological therapy in severe asthma. The presence of respiratory failure that improved so rapidly after the onset of mepolizumab therapy is probably what differentiates it the most from other cases of severe asthma with a favorable response to treatment.