INTRODUCTION
Human and canine allergic diseases have become increasingly common in the last decades. Changes in both the environment and population’s lifestyle, associated with increased exposure to allergens, are factors implicated in their increased incidence1. Canine atopic dermatitis (CAD) is one of the most common allergic skin diseases of dogs, and it is estimated to affect approximately 10-15% of the canine population worldwide2,3. CAD is currently defined as a “genetically predisposed inflammatory and pruritic allergic skin disease with characteristic clinical features associated with IgE antibodies most commonly directed against environmental allergens”4.
In line with what has been demonstrated regarding the induced psychological stress and reduced well-being in human skin diseases, CAD also has a major impact on the quality of life for patients and their cohabiting families5. For dogs, the loss of quality of life is primarily related to the severity of pruritus, the main clinical manifestation of the disease, that often affects the dog’s physiological activities, such as sleeping, walking, or playing6-8. This repeated behaviour damages the skin, perpetuating and increasing the inflammatory process, which in turn induces more pruritus, thus establishing a vicious cycle9. Pruritus is induced by several endogenous mediators, namely interleukins. In 2004, Dillon et al. identified the action of interleukin (IL) 31 in the development of pruritus, alopecia, and skin lesions in mice10. Since then, this interleukin has been used to induce pruritus in dogs experimentally11. Currently, it is known that IL-31 plays a prominent role in the development of pruritus in CAD and its serum levels may be elevated in these dogs9,11,12.
This interleukin is mainly secreted by activated TH2 and memory T lymphocytes and its pruritogenic effect results from the activation of intracellular signalling pathways such as Janus kinase-signal transducer and transcription activator (JAK-STAT), mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)10,11.
Although this interleukin belongs to a cytokine family with important roles in inflammation, immunity, hematopoiesis, development and metabolism, the function of IL-31 in the inflammatory process has been questioned13.
In fact, a recent study demonstrated the action of IL-31 on pruritus and the lack of effect on skin inflammation in cases of contact hypersensitivity14.
Atopic dermatitis is commonly an incurable disease, therefore the ultimate goal of its management is to improve the dog’s quality of life, achieving a reduction in pruritus, a longer interval between flares, as well as a lower degree of secondary complications, such as infections15. For pet owners, ease of drug administration and cost-related issues are also of matter. The International Committee on Allergic Diseases of Animals (ICADA) has updated the guidelines for the treatment of CAD (Treatment of canine atopic dermatitis: 2015 updated guidelines from ICADA), which include the detailed plan for the treatment of acute flares and chronic disease16.
Lokivetmab is the most recent and one of the most promising therapeutic tools for the treatment of CAD’s pruritogenic manifestations. It is the first caninized monoclonal antibody, obtained by recombinant technology from Chinese hamster ovary cell, approved for veterinary medicine use in the European Union in 201717.
Despite this innovative biological therapy, which aims to neutralize canine IL-31, being highly specific, it is also very expensive (average of 90 euros per month, in Portugal).
Moreover, it has an onset of action of only eight hours and a duration of effect of about a month17. Many studies have confirmed the antipruritic effect of this molecule and the therapeutic success associated with its use. Additionally, lokivetmab has a good reputation for safety, with fewer adverse events recorded when compared to other more conventional drugs and with rare induction of antibody-mediated reaction by the organism against lokivetmab18. This monoclonal antibody is, therefore, an advantageous therapeutic option for animals with concomitant systemic or dermatological diseases as it was shown to be effective, well-tolerated and safe19.
The goal of our study was to evaluate the efficacy and safety of lokivetmab (Cytopoint®) in the treatment of dogs with atopic dermatitis and to assess their owners’ overall opinion regarding this therapy.
MATERIALS AND METHODS
Participant enrolment criteria
Eighteen client-owned dogs (irrespective of breed or sex) with perennial atopic dermatitis were selected at the Dermatology Service of the Hospital of the Faculty of Veterinary Medicine of the University of Lisbon and enrolled in the study after the owners’ written informed consent has been obtained.
All dogs had undergone standard diagnostic evaluation and fulfilled criteria for the clinical CAD diagnosis, based on the history, clinical signs and according to the recommended criteria by Favrot20. All differential diagnoses were excluded, namely the possibility of external parasites, by establishing an antiparasitic protocol for each animal, which was maintained throughout the trial. Maintaining other concomitant pre-established medications (including antibiotics, antifungals, ectoparasiticides, fatty acids, vitamin E and therapeutic shampoos) was allowed, provided they had been instituted before the start of lokivetmab and the dosing regimen was maintained during the trial. Allergen immunotherapy was permitted if it had been used for more than twelve months, in an unchanged dose for six months, and the regimen was maintained throughout the study.
Exclusion criteria included the interruption of treatment before the end of the study, the non-compliance of the owners in filling out the scale for the classification of pruritus, the lack of attendance to the proposed evaluation consultations and pregnant/lactating bitches.
This study was approved by the Faculty of Veterinary Medicine’s Ethics and Animal Welfare Committee.
Study protocol
This study was designed as an eight-week clinical trial. Throughout the study period (56 days), dogs have received two subcutaneous administrations of lokivetmab, on days 0 and 28, with the appropriate dose for their weight, according to the licensed dosage of the drug (Figure 1).
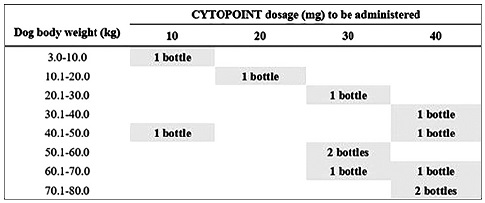
Figure 1 Description of the dose of lokivetmab (Cytopoint®) to be administered depending on the dog’s body weight. Recommended minimum dose: 1 mg/kg. (Image adapted from European Medicines Agency 2018).
The efficacy of lokivetmab was determined by the clinical evolution of the skin lesions, assessed by the veterinarian, using the validated Canine Atopic Dermatitis Extent & Severity Index (CADESI)-04 scale, and by the quantitative evaluation of the pruritus by the owners, using the validated Pruritus Visual Analogue Scale (PVAS). The evaluation of skin lesions occurred at four stages in the hospital, on days 0, 14, 28, 56. The pruritus score was assigned 14 times, on days 0, 1, 2, 3, 4, 5, 6, 13, 20, 27, 34, 41, 48 and 55, in a notebook provided to the owners for that purpose.
The safety of the treatment was assessed using analytical parameters of liver function (ALT - alanine aminotransferase - and ALP - alkaline phosphatase) and blood count, with blood sampling performed on days 0 and 56. Besides, owners were provided with a diary to record potential adverse effects presented by the animals.
The general owners’ satisfaction regarding the treatment was evaluated during the follow-up visits and based on the number of animals that maintained the therapy after the end of the study.
Statistical analysis
The collected data were organized in the Microsoft Office 365 program, Excel® (version 16.19) and the statistical analysis was performed using the R® program (version 3.5.1). Pruritus’ serial classifications were assessed using the Kaplan-Meier method. To compare some of the curves obtained by this method, the non-parametric Log-Rank test was used. To assess the difference between the pruritus and CADESI-04 values at diferente times, the Wilcoxon non-parametric test was used for paired samples that do not follow a normal distribution.
For all analysis, values of p<0.05 were considered statistically significant. Confidence intervals (CI) were calculated using the Wilson method of the Epitools Epidemiological Calculators website, developed by Ausvet®.
RESULTS
Pruritus score evolution
Due to lack of sufficient data, one of the dogs had to be excluded from the sample prior to the results’ analysis.
The pruritus score values recorded on day 0 had a median of 7. After four weeks (day 27), the median pruritus score decreased to 4. In the last evaluation, eight weeks after the start of treatment (day 55), the median pruritus score had been reduced to 3.
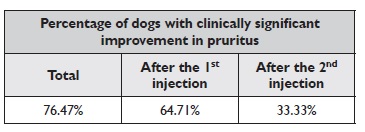
Considering a reduction of at least 50% in the pruritus score value as a clinically significant improvement, this was observed in 76.47% of the dogs, 95% CI [52.74%- 90.44%], over the eight-study weeks (between days 0 and 55). If we only consider the first four weeks (between days 0 and 27), there was a clinically significant improvement in the pruritus in 64.71% of the dogs, 95% CI [41.30%-82.69%]. Of those who did not significantly improve after the first injection of lokivetmab, 33.33% (95%
CI [9.68%-70%]) improved in the following four weeks.
The results of the efficacy of lokivetmab in reducing the dog’s pruritus over the eight weeks and after the first and second injections are summarized in Table 1. Although clinically significant improvement was not achieved by all animals (n=4), there was a consistent reduction in the pruritus value in 75% of these dogs (n=3) over the study time, 95% CI [30.06%-95.44%].
Table 1 Percentage of dogs that achieved clinically significant improvement in pruritus over 8 weeks (total), after the first injection and after the second injection of lokivetmab
The pruritus score values’ reduction between day 0 and day 55 was statistically significant (W=125, p=0.023), as well as the decrease in the value in the first four weeks, between day 0 and 27 (W=90, p=0.02). It was also observed that more than 50% of the dogs with clinically significant improvement reached it before the seventh day after the first injection.
Lesional score evolution
The CADESI-04 values in the first evaluation (day 0) had a median of 23.5. After four weeks, on the second injection of lokivetmab, the median value decreased to 12. In the last evaluation, eight weeks after the start of treatment (day 56), the median value was 12.5.
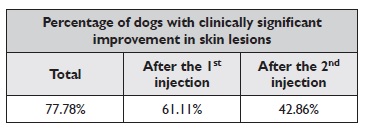
As with pruritus, a reduction of at least 50% in the CADESI-04 value was considered to be clinically significant. During the total eight-weeks period, there was an improvement in 77.78% of the dogs, 95% CI [54.79%-91%]. If we only take into account the first four weeks (between days 0 and 28), there was a clinically significant improvement in 61.11% of the animals, 95% CI [38.62%-79.69%]. Of those who did not improve after the first injection of lokivetmab, 42.86% improved in the following four weeks (after the second injection), 95% CI [15.82%-74.95%]. The results of the efficacy of lokivetmab in reducing dog’s skin lesions over the eight weeks and after the first and second injections are summarized in Table 2. Of the animals that did not achieve a clinically significant improvement in the lesional score (n=4), 75% (n=3), 95% CI [30.06% -95.44%] recorded a decrease in the CADESI-04 value over the study period. The reduction in CADESI-04 values between day 0 and day 56 was statistically significant (W=150, p=0.005).
Table 2 Percentage of dogs that achieved clinically significant improvement in skin lesions over 8 weeks (total), after the first injection and after the second injection of lokivetmab
There was also a statistically significant difference between days 0 and 14 (W=108, p=0.007) and between day 0 and day 28 (after four weeks of the first injection) (W=148.5, p=0.006). However, the decrease in CADESI-04 values between four weeks after the start of treatment (day 28) and the last evaluation (day 56) was not statistically significant (W=78, p=0.96).
Adverse effects and laboratory analysis
During the eight weeks, there were two episodes of vomiting in different animals and one episode of vomiting and diarrhoea in another dog. None of these events required medical intervention. Regarding blood tests, no clinically significant changes were detected in the results of the second collection (after two administrations of lokivetmab).
Although the development of antibodies against lokivetmab has not been investigated, there were two animals (11.76%, 95% CI [3.29%-34.34%]) whose progression of the pruritus over the two months could be compatible with this situation.
Follow-up after project completion
Upon study completion, 14 dogs (77.78%, 95% CI [54.79%-91%]) continued to receive monthly lokivetmab administrations, as part of CAD’s therapeutic control, for at least the following three months.
DISCUSSION
Evaluation of the pruritus score evolution
Pruritus progressed positively over time, with a reduction that proved to be statistically significant both at the end of the first (day 27) and second months (day 55), concerning the beginning of treatment (day 0). The median pruritus value decreased more than half, with just two injections of lokivetmab.
The decision to consider the criterion of clinically significant improvement only for value reductions of at least 50%, to assess the efficacy and treatment success with lokivetmab, was based on its previous use in several similar clinical studies, as in the works of Michels and his collaborators and Cosgrove et al., respectively related to lokivetmab and oclacitinib12,18,21. The use of this criterion was an attempt to obtain more demanding results regarding the efficacy of lokivetmab. Consequently, the results suggest the efficacy of lokivetmab in reducing pruritus in most animals (76.47% during the total time understudy).
This value is similar to that obtained (77%) by Souza and collaborators in their retrospective study regarding the use of lokivetmab in allergic pruritus’ management in dogs19. ICADA has recently developed a Core Outcome Set for therapeutic clinical trials enrolling dogs with CAD (COSCAD’18)22. According to these new recommendations, the pruritus outcome measure would be the percentage of dogs with owner-assessed pruritus score in the range of normal dogs or the mild CAD range at the end of the study22. If this therapeutic success criterion had been adopted in this study, better results would probably have been obtained. Namely, a greater number of animals with clinically significant improvement in the pruritus score would have been found.
The effect of lokivetmab in pruritus’ reduction was evident in the first 24 hours (at the day-1 evaluation), which is in line with the results of other studies18,19. It was possible to notice from the owners’ report that, in some cases, lokivetmab took effect a few hours after its administration. Moreover, before the seventh day after the first injection, a clinically significant improvement had already been achieved in more than half the animals.
Although a clinically significant improvement, according to our established criteria, was not achieved by all animals, all but one dog showed a decrease in the initial pruritus score. This only dog already had a pruritus score within the normal range (2 out of 10) to begin with, as he had been treated with oclacitinib before its inclusion in this study. To be able to provide such a comfortable level of pruritus under Cytopoint® therapy was, therefore, considered a therapeutic success.
The fact that some animals (n=4) did not achieve satisfactory results can be justified for several reasons. The formation of antibodies against lokivetmab, although it was not tested in this study, could explain two (11.76%) of these cases, where the pruritus score increased after the second administration. Despite being caninized antibodies, there is the possibility of creating antibodies directed against the 10% hamster antibody residue, which would neutralize the lokivetmab effect17,23. These antibodies were researched and quantified in a 2016 study, which evaluated this molecule’s safety, and were found in 2.5% of the animals that received the treatment12. Another study, from 2018, found loss of pruritus control after the second injection of lokivetmab in 2.6% of the animals under treatment. In the latter, antibodies directed against lokivetmab were not researched. However, the authors considered this a plausible hypothesis to justify the results19. Additionally, the lack of response to lokivetmab or the less pronounced response may also be related to the complexity of this syndrome and the combination of multiple pruritus mediators involved in the CAD pathogenesis, with a predominance of diferente mediators over IL-319.
Evaluation of the skin lesions score evolution
The progress of CADESI-04 classifications was satisfactory, with a statistically significant reduction in values between the day of the first administration (day 0) and the final of the study (day 56). The decrease in these values was observed shortly after two weeks since the first injection. The reduction was also statistically significant both between days 0 and 14 and between days 0 and 28. The median skin lesion value decreased by almost half, with only two injections of lokivetmab.
If we consider the same criterion used for the pruritus score, this study suggests that lokivetmab is eficiente in reducing skin lesions since there was a clinically significant improvement in the CADESI-04 value in 77.78% of the animals over the total period under study. In the first month, 61.11% of the dogs reached a clinically significant improvement, a value higher than those recorded by Michels et al. in a similar time interval but based on CADESI-03 (46% in the dog group receiving 2 mg/kg of lokivetmab and 22% in the group of 0.5 mg/kg)18.
According to COSCAD’18, the lesional outcome to be considered would be the percentage of dogs with veterinarian-assessed skin lesions in the range of normal dogs or the mild CAD range at the end of the study22. Once again, if this criterion had been assumed in this study, better results regarding a greater number of dogs with clinically significant improvement in the lesional score would probably have been reported.
Similarly to what was observed in pruritus, despite an improvement of at least 50% in the value of CADESI-04 not being seen in all animals, there was a reduction in its initial value in all dogs except one. Nevertheless, the CADESI-04 values of this last animal have always remained low, in the interval classified as mild, throughout the study period. Looking at intervals for CAD severity classification using CADESI-04 values (<10: normal dogs or remission, 10-34: mild CAD, 35-59: moderate CAD, 60: severe CAD)24, it is possible to verify that the majority of dogs were classified within the mild range, in the first evaluation (day 0), with only one severe case and three moderate cases. In the following clinical evaluations, no severe cases were registered. In the last evaluation (day 56), most animals were in the mild range, with only one moderate case and six animals evolving to the remission group.
Therefore, lokivetmab was found to be effective in reducing CADESI-04 values for most animals, and in maintaining low scores between administrations (for at least four weeks). The effect of lokivetmab in pruritus may have favoured the improvement of skin lesions, by interrupting the itching and the inflammation - pruritus vicious cycle. While pruritus decreases rapidly with treatment, skin lesions may take longer to resolve. Therefore, in short-term studies, as the current one, the evaluation of the effect of a therapeutic approach may not fully represente reality.
Adverse effects and laboratory analysis assessment
During the two months, only three cases of adverse events were recorded. The three animals presented episodes of vomiting, and one of them also had diarrhoea.
In all cases, the situation was resolved without the need for intervention. These side effects in animals being treated with lokivetmab have been described in the drug’s safety study. Lethargy and vomiting were the most common adverse reactions observed by Souza and collaborators, in their retrospective study in 2018, and in the present study, situations were solved without the need for medical intervention19.
Regarding physical examinations and blood tests performed before and after treatment, there were no changes with clinical significance. These observations are in line with the results obtained in the study by Michels et al. in which no analysis’ changes recorded in animals treated with lokivetmab12. Although drug interactions were not actively investigated, they were not observed, similarly to what was seen in a previous study12.
The absence of interaction with other medications and the specific action to a single interleukin suggest that lokivetmab may be an excellent option for dogs with concomitant diseases that make treatment with other molecules unfeasible. More specifically, in case of dogs with neoplasia to which oclacitinib must not be administered.
The results of this study indicate a high safety standard for lokivetmab, with no record of serious adverse effects or the need for veterinary intervention.
General owner’s satisfaction and treatment maintenance after project completion CAD’s emotional and physical challenge for the owners, as well as the implications of its management, both financially and in the time invested, greatly influence their quality of life5,8. Owners’ satisfaction regarding the therapeutic plan is, therefore, paramount to maintain their compliance and, ultimately, achieve therapeutic success7.
The owners’ opinion was inquired during the follow- up consultations and a high satisfaction’s degree was found regarding the lokivetmab clinical results. This biological therapy has the great advantage of controlling the patients’ clinical condition over about a month, with just one injection. Since it does not require daily home drug administration, which could lead to stress for both owners and dogs and ultimately deteriorate their relationship25, this therapeutic option proves to be very conveniente and attractive for many owners. The high number of dogs that, after three months from the end of the study (77.78%), maintained treatment with lokivetmab reflects the most-owners’ satisfaction. Moreover, some of the owners testified that lokivetmab provided a great improvement in their dogs’ and families’ quality of life. There were only four owners who did not notice a significant difference with the use of lokivetmab in their animals.
Two of these dogs were under treatment with other molecules before the study started, and, in the owners’ opinion, lokivetmab has achieved similar control of clinical signs compared to previous treatment. For economic reasons, these four animals returned to their previous therapy at the end of the study.
Long-term treatment with lokivetmab turns out to be costly and unaffordable for some owners, who have to choose other treatment options to best suit their financial condition. Even so, in this Portuguese Dermatology Service, since April 2018, an average of twenty-five patients are undergoing monthly treatment with Cytopoint®.
Study limitations and future research Ideally, to assess the effects of lokivetmab, no other medication should have been simultaneously used. However, the main concern of this project was the welfare of the animals and, given the real clinic scenario and the severe clinical presentation of some cases, there was a need to implement a multimodal and individualized therapeutic plan. In order to avoid this inconvenience, all medications used concurrently with lokivetmab were registered and administered to interfere as minimum as possible with the results.
The small sample size was a limiting factor, making certain investigations impossible, such as comparing the efficacy of lokivetmab among different breeds and between animals with high and low initial pruritus values.
Larger future investigations could fruitfully explore these issues further. The owner’s motivation in filling out the scales for pruritus assessment also limited the sample size, resulting in the exclusion of some dogs from the study, whose owners did not respect the number of requested evaluations. Besides, although a detailed and described scale was provided to all owners for determining pruritus’ value at each evaluation time, the interpretation and consistency of its filling may vary between people and by the same person over time. Lokivetmab cost, considered by many owners to be high and beyond their financial means, was also a limiting factor for the inclusion of animals in the study.
Although the clinical efficacy and safety of this biological therapy have been described, long-term studies to assess these issues as a chronic treatment approach are urgently needed. Furthermore, despite a recent study has demonstrated that Lokivetmab can be used as a proactive regimen for pruritus control (and not successfully for lesion’s prevention), further studies are welcome to identify the most likely responder dogs26. Moreover, although treatment-induced immunogenicity has been discussed in previous works, further research on this topic would provide insight into the clinical relevance of this finding and whether it interferes with the long-term treatment response rate.
CONCLUSIONS
The use of lokivetmab proves to be an extremely effective and safe strategy for controlling CAD. The significant clinical reduction in pruritus and skin lesions scores, the absence of relevant side effects, and the great specificity directed at IL-31 make this therapeutic tool one of the most potent and promising in the CAD’s field.
Furthermore, since it spares daily home drug administration and allows for a month to control the patient’s pruritus, the use of lokivetmab becomes very convenient and leads to a high owner’s satisfaction degree. Nevertheless, its high cost associated with the need to maintain longterm treatment may, in some cases, restrict its adoption, even when the therapeutic success and owners’ satisfaction are high.















