Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.1 Lisboa jan. 2012
Iron isomaltoside 1000: a new high dose option for parenteral iron therapy
Philip A. Kalra1, Klaus Bock2, Morten Meldal3
1 Department of Renal Medicine, Salford Royal Hospital, Salford, United Kingdom.
2 Department of Chemistry, University of Copenhagen, Copenhagen, Denmark.
3 Nano Science Center, University of Copenhagen, Copenhagen, Denmark.
ABSTRACT
Iron isomaltoside 1000 (Monofer®) is a new dextran-free parenteral iron product, currently approved in 22 EU countries. Iron isomaltoside 1000 consists of iron and a carbohydrate moiety where the iron is tightly bound in a matrix structure, which enables a controlled and slow release of iron to iron-binding proteins, avoiding toxicity. The carbohydrate,isomaltoside 1000, is a purely linear chemical structure with low immunological activity. Due to the structure of iron isomaltoside 1000 and the low anaphylactic potential, there is no requirement for a test dose, and it can be administered in high doses with a maximum dosage of 20 mg/kg within 30-60 minutes in one visit. Thus, iron isomaltoside 1000 offers the broadest dosage range compared to other parenteral iron products on the market.
Due to the dose flexibility, the possibility of providing full iron correction in one single visit makes iron isomaltoside 1000 highly convenient for both the health care professional and the patient. Clinical studies of iron isomaltoside 1000 show that it is an effective and well-tolerated treatment of iron deficiency anaemia with a favourable safety profile.
Furthermore, iron isomaltoside 1000 does not seem to induce hypophosphataemia.
Key-Words:High dose; iron deficiency anaemia; iron isomaltoside;iron treatment.
INTRODUCTION
The ability to give high doses of iron is important in the management of iron deficiency anaemia (IDA) in a number of clinical conditions where demands for iron are high, such as chronic kidney disease (CKD), chronic blood loss associated with inflammatory bowel disease (IBD) or other gastrointestinal disease, pregnancy, and blood loss during surgery.Parenteral iron offers a fast iron correction, and it is superior to oral iron in many circumstances, especially in the treatment of anaemia associated with chronic diseases where the patients may be intolerant of oral iron or because the iron absorption may be blocked, in cases with large iron deficits as the maximum capacity for oral iron absorption is very limited, or when patients are treated with erythropoiesisstimulating agents (ESAs).
The currently available parenteral iron preparations include high molecular weight iron dextran (Dexferrum ®), low molecular weight iron dextran (Cosmofer®/Infed®), iron gluconate (Ferrlecit®), iron sucrose (Venofer®), ferumoxytol (Feraheme®), iron carboxymaltose (Ferinject®/Injectafer®), and iron isomaltoside 1000 (Monofer®). They are generally considered equally efficacious, but most of them have limitations in dosing, administration (duration and frequency), and safety profile. High molecular weight iron dextran has been associated with an increased risk of anaphylaxis/anaphylactoid reactions and is not available in Europe1. These side effects are significantly reduced with low molecular weight iron dextran1, but this still requires a test dose and has a long infusion time of four to six hours for larger doses6. Iron gluconate, iron sucrose, and ferumoxytol (only available in US and use limited to CKD patients) can only be administered in moderate doses since they are limited to a maximum total single dose of 125 mg, 200 mg, and 510 mg, respectively7-9. In addition, treatment with iron sucrose requires a test dose in Europe8, and it has been found associated with acutely increased proteinuria at 100 mg weekly infusions10,11. Iron gluconate has also been found to be associated with a mild transient proteinuria in CKD patients11.
Iron carboxymaltose does not require a test dose, and it can be administered in doses of 20 mg/kg up to a maximum of 1000 mg per infusion12. Iron carboxymaltose infusion has been associated with hypophosphataemia of unknown aetiology. The clinical significance of the hypophosphataemia is unknown13.
The newest parenteral iron preparation, iron isomaltoside 1000 (Monofer®), was introduced in Europe in 2010. The ambition with iron isomaltoside 1000 was to develop an efficacious parenteral iron product with a favourable safety profile without test dose requirement and without dose limitations in order to optimise dosing flexibility and user convenience. Iron isomaltoside 1000 fulfils these requirements and can be administered with a maximum dosage of 20 mg/kg, no test dose, and within 30-60 minutes in a single visit13. Due to the dose flexibility
that iron isomaltoside 1000 offers, the possibility of providing full iron correction in a single infusion makes it highly convenient for both the health care professionals and the patient. The present review describes the physiochemical characteristics, pharmacological, pharmacokinetic, and immunogenic properties, preclinical and clinical data, and cost analysis of iron isomaltoside 1000.
PHYSIOCHEMICAL CHARACTERISTICS OF IRON ISOMALTOSIDE 1000
Iron isomaltoside 1000 consists of iron and a carbohydrate moiety. The carbohydrate isomaltoside 1000 consists predominantly of 3-5 glucose units and originates from isomalto-oligosaccharides produced by hydrolysis of dextran, followed by subsequent fractionation and chemical modification to provide a product with the desired molecular weight distribution. Furthermore, isomaltoside 1000 is isolated after chemical reduction of the reducing sugar residues to avoid complications due to redox reactions or degradation of the aldehyde group at the anomeric centre. The absence of reducing sugar prevents any complex redox reactions and thereby degradation of the iron complexes16. Apart from differences in molecular weight between dextran in iron dextran and isomaltoside 1000, the latter is also completely devoid of any branching structures as evidenced by 13C and 1H NMR spectroscopic analysis and it does not contain any reducing sugar residues15. Thus, although isomaltoside 1000 is manufactured by a chemical modification and hydrolysis of dextran, isomaltoside 1000 is not a dextran. The chemical structure of isomaltoside 1000 is very different from the dextran structure, in which the α-(1,3) ed branches of the molecule are wound around the main chain α-(1,6) ed polymer in a tight helical arrangement15 while isomaltoside 1000 has a purely linear oligomer structure of α-(1,6) ed glucopyranose residues, on average repeating 5.2 times, and contains no reducing sugar units.
Electron microscopy16 presented the nano structure of iron isomaltoside 1000 as spheroidal while 13CNMR and associated molecular modelling have indicated that it is composed of a matrix structure in which the iron atoms are predominantly bound and dispersed in the matrix. From the masses of the components it can be calculated that there are approximately 10 iron atoms bound per oligosaccharide molecule. It is not yet known if these constitute coordinated single iron oxide moieties or small clusters of coordinated iron oxide. The iron isomaltoside 1000 matrix is composed of interchanging strands of linear isomaltoside 1000 with iron atoms placed in cavities between and within the isomaltoside molecules15. The matrix structure enables a controlled and slow release of iron which attaches to iron-binding proteins with little risk of free iron toxicity (Fig. 1). This is a unique structure and quite different from other iron products which are described as a pure iron core surrounded by a carbohydrate shell. The formation of this molecular matrix structure is possible due to the short, linear, and non-ionic isomaltoside 1000 structure combined with the production technology for complexing iron and isomaltoside 1000.
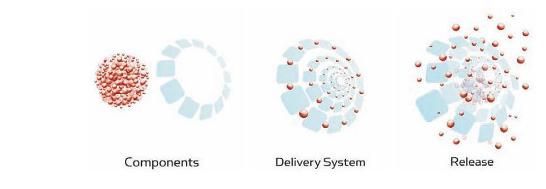
Figure 1
Matrix structure of iron isomaltoside 1000 wich enables a controlled and slow release of iron.
Iron is tightly bound in the iron isomaltoside 1000 molecule; assessment of an iron isomaltoside 1000 solution equivalent to that administered to patients showed very low concentrations of free iron close to the detection limit of the assay (Fig. 2). A similar low concentration of free iron has been found with ferumoxytol, while the concentration of free iron in iron dextran and iron carboxymaltose solutions is significantly higher15. Measurement of labile iron showed that the newer iron products (iron carboxymaltose, ferumoxytol, and iron isomaltoside 1000) have low labile iron content when compared to the older products (iron dextran << iron sucrose and iron gluconate)15.
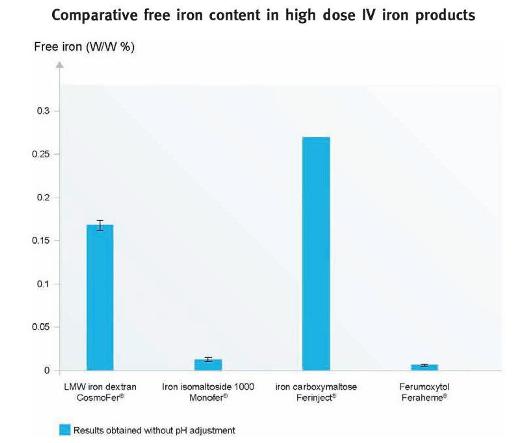
Figure 2
Free iron content in high dose parenteral iron products. The data is obtained without pH adjustment. The detection limit was 0.002 %. [Modified from Jahn et al.2011 (Ref.16)].
I MMUNOGENIC PROPERTIES OF ISOMALTOSIDE 1000
Anaphylactoid/anaphylactic reactions may occur with all parenteral iron compounds and were seen relatively often with the old high molecular weight iron dextran products. The pathogenic mechanisms for these reactions is not entirely clear, but the reactions seem to occur both through specific and non-specific immune reactions, with the carbohydrate carrier playing an important role for these reactions16. Thus, an important aim of the development of iron isomaltoside 1000 was to develop a product with a low risk of anaphylactoid/anaphylactic reactions. In order to achieve this, a carbohydrate carrier with a low immunogenic potential was sought. In iron isomaltoside 1000, the carbohydrate carrier, isomaltoside 1000, is based on a chemical modification of oligomers known to prevent dextran-induced anaphylactic reactions. Since it is well known that homopolymers of glucose have very low immunogenic potential17, in product design, any residual branching units that were α-ed to the 3-position of the main chain were removed, and the reducing sugar residue was chemically transformed quantitatively to non-reducing groups.
Thus, from a theoretical point of view, the immunogenic potential of iron isomaltoside 1000 is expected to be very low, and on this basis, the regulatory authorities decided that no test dose was required in the first clinical studies with iron isomaltoside 1000. These studies supported the theoretical rationale for low immunogenic activity, and iron isomaltoside 1000 was therefore approved for use without a test dose.
PHARMACOLOGICAL AND PHARMACOKINETIC PROPERTIES OF IRON ISOMALTOSIDE 1000
Following IV administration, iron isomaltoside 1000 is rapidly taken up by the cells in the reticuloendothelial system (RES), particularly in the liver and spleen, from where iron is slowly released. The plasma half-life is 5 hours for circulating iron and 20 hours for total iron (bound and circulating). Circulating iron is removed from the plasma by cells of the RES which split the complex into iron and isomaltoside 1000. Iron is immediately bound and stored, mainly in ferritin. The iron replenishes haemoglobin and depleted iron stores. Negligible quantities of iron are eliminated in the urine and faeces. Due to the size of the nanoparticles (20.5 nm), iron isomaltoside 1000 is not eliminated via the kidneys.The carbohydrate component, isomaltoside 1000, is either bolised or excreted unchanged via the kidney14.
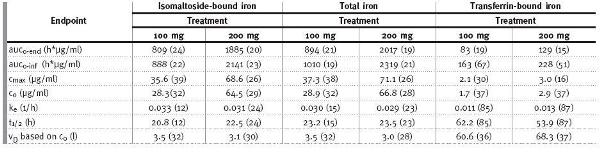
An open-label, cross-over, single-centre study was performed in 12 patients (5 men/7 women) with inflammatory bowel disease (IBD) to assess pharmacokinetics18. The patients were allocated to one of two single-dose treatments where iron isomaltoside 1000 was administered as a single bolus dose of 100 or 200 mg with a four-week interval between the two doses. The dose was administered at a maximum of 50 mg of iron/minute. Pharmacokinetic (PK) variables were analysed for total iron (TI), isomaltoside-bound iron (IBI), and transferrinbound iron (TBI) according to a one-compartment model. TI and TBI were measured by the Graphite GFAAS system and the Advia chemistry system, respectively, and IBI was calculated by subtracting TBI from TI, assuming that no free iron was present and that quantities of ferritin were negligible, so that the only iron forms present in plasma were TI, TBI, and IBI. The concentration versus time relationship for IBI and TI showed first-order kinetics with small deviations for dose-linearity, and the PK parameters for IBI were close to that of TI (Table I). Thus, TI could be used as a marker of iron isomaltoside 1000 PK in future PK studies. Only approximately 1 % of the doses administered were excreted in the urine. One of the patients was withdrawn after receiving a 100 mg dose because of abdominal pain and flushing. No serious adverse events (SAE) were reported18.
Table I
Geometric mean (CV in %) for PK parameters of IBI, TI, and TBI
Presently, there are several PK studies ongoing with higher doses of iron isomaltoside 1000 in different patient populations (ClinicalTrial.gov: NCT01213979, NCT01280240, NCT01213992, NCT01469078, and NCT01213680).
EFFICACY AND SAFETY PROFILE OF IRON ISOMALTOSIDE 1000
In the recent past, the efficacy and safety of iron isomaltoside 1000 in the treatment of IDA has been investigated in two phase III clinical studies in patients with either chronic kidney disease (CKD) or chronic heart failure (CHF)19,20. The primary endpoint of these studies was to establish the safety profile of iron isomaltoside 1000, whereas efficacy was the secondary endpoint. Both were open-label, noncomparative, multi-centre studies where the patients attended six visits during a study period of eight weeks. At the investigators discretion, iron isomaltoside 1000 was administered either as four repeated intravenous (IV) bolus injections with 100-200 mg iron per dose administered at baseline and at week 1, 2, and 4 (the last dose could be administered as total remaining dose if the total calculated iron requirement exceeded 800 mg) or as a high single iron correction dose (total dose infusion (TDI)) at baseline. If the TDI requirement exceeded 20 mg iron/kg the dose was divided into two and these given at an interval of one week. No test dose was given. The total calculated iron requirement and administered cumulative dose in each patient were based on a target Hb of 130 g/L and utilising the Ganzoni formula that reflects body weight, the difference between actual haemoglobin and target haemoglobin, and the desired level of iron stores (commonly 500 mg)21. The safety assessments consisted of type and frequency of adverse events (AEs) and SAEs, changes in vital signs (including electrocardiogram (ECG)), and clinical laboratory analyses (biochemistry: s-sodium, s-potassium, s-creatinine, s-albumin, s-urea, s-bilirubin, and alanine aminotransferase (ALAT), haematology: leucocytes, complete blood cell count with differentials, and platelets) 1, 2, 4, and 8 weeks after baseline. The efficacy assessments consisted of laboratory monitoring of treatment effect on haemoglobin (Hb), transferrin saturation (TSAT), and s-ferritin levels 1, 2, 4, and 8 weeks after baseline. In addition, the CHF study included s-iron, which was monitored at the same time points, and a linear analogue scale assessment (LASA) quality of life (QoL) questionnaire measuring QoL 4 and 8 weeks after baseline. The LASA is a validated QoL assessment consisting of 100-mm linear analogue scales that measured the patients energy level, ability to do daily activities, and overall QoL.
I RON ISOMALTOSIDE 1000 ADMINISTERED TO PATIENTS WITH CHRONIC KIDNEY DISEASE
The study was conducted at 15 centres in three European countries (six in Denmark, seven in Sweden, and two in the United Kingdom). A total of 182 CKD patients (128 men/54 women) receiving dialysis (n =161) or pre-dialysis care (n = 21) were included. The vast majority of patients were receiving haemodialysis. The patients were generally on ESA treatment (n = 82 %), and the dosage of ESA was kept constant during the study. Patients were either switched from an existing parenteral iron maintenance therapy (n = 144) or were not currently treated with parenteral iron (n = 38). The mean ± SD age was 63.3 ± 13.8 years (range: 21-91 years). Patients not receiving parenteral iron treatment when entering the study had a baseline Hb of 99.1 ± 9.0 g/L and a s-ferritin of 231 ± 154 μg/L, and patients who switched from a parenteral iron maintenance regimen had a baseline Hb of 114.9 ± 10.3 g/L and a s-ferritin of 380 ± 195 μg/L. The mean ± SD total cumulative dose of iron per patient was 529 ± 283 mg19. In total, 584 treatments were given (523 IV bolus 100 mg, 17 IV bolus 100-200 mg and 44 TDIs) with single doses as high as 1800 mg22.
Nineteen reported AEs were possibly or probably related to the study drug. There was no difference in the AE frequencies observed in patients treated with bolus doses or TDI. Three subjects (1.6 %) had more than one AE related to the study drug: one patient had two events of nausea, another patient had diarrhoea, influenza, hyperhidrosis, low s-ferritin, and arthralgia, and a third patient had a haemorrhagic cyst in the right kidney and pruritus. Two of the AEs which were determined by the attending physician to be possibly treatment-related, fulfilled the criteria for SAEs. The events were sepsis with Staphylococcus aureus and unstable angina. Two deaths (one reported as due to an unknown cause and the other pneumonia) occurred, but these were both considered unrelated to the study drug. No acute anaphylactoid/anaphylactic or delayed allergic reactions were reported. There were no clinically significant changes in vital signs or routine safety clinical laboratory tests.
Hb increased from 99.2 ± 9.0 g/L at baseline to 111.2 g/L ± 14.7 at week 8 in patients not having received parenteral iron (p < 0.001) and remained stable in patients receiving maintenance iron therapy (114.9 ± 10.3 g/L at baseline, 117.5 ± 11.7 g/L at week 8; p = 0.05). The mean ± SD maximal increase in Hb in the overall mixed CKD population was 7.9 ± 9.9 g/L (p < 0.001). TSAT and s-ferritin also increased significantly from baseline to week 8 (p < 0.001). It was concluded that iron isomaltoside 1000 administered to CKD patients as repeated bolus injections or TDI without a test dose, was safe and well-tolerated and resulted in improved markers of iron status and anaemia19.
I RON ISOMALTOSIDE 1000 ADMINISTERED TO PATIENTS WITH CHRONIC HEART FAILURE
The study was conducted at seven centres in two European countries (four centres in Denmark and three centres in Sweden). A total of 20 CHF patients (10 men/10 women) with mild anaemia were included. None of the patients received ESA treatment. The mean age was 75 years (range: 61-88 years). Baseline Hb was 108.2 ± 7.6 g/L and s-ferritin was 180 ± 184 μg/L. All 20 patients received a high single dose infusion with a mean infusion time of 59.8 minutes (range: 50-67 minutes) with a mean dose of 868 mg (range: 650-1000 mg).
No study drug related AEs were reported, no deaths occurred, and no acute anaphylactic/anaphylactoid or delayed allergic reactions were observed. There were no clinically significant changes in routine clinical safety laboratory tests or vital signs. New clinically significant ECG abnormalities were observed on 13 occasions, but these did not indicate any new disease or progression of disease and could all be explained by the patients medical history.
Haemoglobin was increased at every visit compared to baseline; however, the increase was nonsignificant due to the small patient population. As compared with baseline value, s-ferritin was significantly increased at all visits, while a statistical increase in s-iron and TSAT were observed one week after baseline. All QoL assessments increased significantly four weeks after baseline. The empirical mean for energy level increased by 49 %, ability to do daily activities increased by 38 %, and overall QoL increased by 23 %. Eight weeks after baseline, the scores were increased by 34 %, 20 %, and 13%, for each of these QoL parameters, respectively, but statistical significance was only reached for energy level. The authors concluded that, despite the uncontrolled study design and small sample size, iron isomaltoside 1000 was well-tolerated and improved QoL in patients with CHF20.
HIGH DOSING OF IRON ISOMALTOSIDE 1000
As discussed, iron isomaltoside 1000 can be administered in high doses without a test dose due to its low immunological activity and low risk of free iron related toxicity. Three sub-analyses of safety and efficacy parameters of the patients in the CKD and CHF studies, who were treated with high dose infusion, were performed23-25.
The first analysis included 19 haemodialysis patients with CKD and anaemia. All 19 patients received a high single dose infusion, with a mean dosage of 986 mg (range: 463-1800 mg) over 30-60 minutes. A total of 19 AEs were reported in 9 patients (47 %), but none of them was considered to be related to the study drug by the investigator. No acute anaphylactoid/anaphylactic or delayed allergic reactions were observed, and there were no significant changes in safety clinical laboratory tests or vital signs. Efficacy markers of IDA improved significantly23.
The second analysis included 21 pre-dialysis patients with CKD and anaemia. One SAE that was considered to be treatment related was observed. The event was angina pectoris in an 80-year-old CKD patient with a medical history of angina. It occurred 10-11 days after the patient had received 1400 mg iron isomaltoside 1000. The medical history of the patient and the time delay in the occurrence of the event made the relationship of the SAE to iron isomaltoside 1000 quite unlikely. No acute anaphylactoid/anaphylactic or delayed allergic reactions were observed, and there were no significant changes in safety clinical laboratory tests or vital signs. Efficacy markers of IDA improved significantly25.
The third analysis consisted of the above 40 CKD patients aggregated with the 20 CHF patients. A total of 58 out of 60 patients had one single high dose infusion, and only two CKD patients required two divided doses in order to fulfil their iron needs. The mean dosage was 975 mg (range: 462-1800 mg) in the CKD patients and 868 mg (range: 650-1000 mg) in the CHF patients. One treatment related SAE was observed, which was the event of angina pectoris discussed above. No acute anaphylactoid/anaphylactic or delayed allergic reactions were observed, and there were no significant changes in safety clinical laboratory tests or vital signs. Efficacy markers of IDA improved significantly24.
In conclusion, iron isomaltoside 1000 administered as high doses to CKD and CHF patients was safe, well tolerated, and effective in improving markers of IDA.
I RON ISOMALTOSIDE 1000 AND NEPHROTOXICITY
It has been suggested that parenteral iron may have a direct toxic effect on renal tubular cells which could cause renal phosphate wasting26,27. In 2004, Zager and colleagues reported a study comparing the nephrotoxicity of iron sucrose, iron gluconate, iron dextran, and iron isomaltoside 1000 over a broad dosage range (0, 30 to 1000 μg iron/mL)28. The iron preparations were added to isolated mouse proximal tubule segments and cultured proximal tubular human kidney cells. Cell injury was assessed by lactate dehydrogenase release, adenosine triphosphate reductions, cell cytochrome c efflux, and/or electron microscopy. The iron preparations evoked in vitro toxicity and up to 30-fold differences in severity were observed. The highest toxicity was observed in iron sucrose and the lowest in iron dextran and iron isomaltoside 1000 (iron sucrose > iron gluconate >> iron dextran = iron isomaltoside 1000). The large differences may be explained by the difference in capacities of the irons to gain intracellular access. According to transmission electron microscopy (TEM) studies, the nanostructure of iron dextran and iron isomaltoside 1000 are similarly large and globular while iron sucrose and iron gluconate, in addition to being more soluble formulations, differ significantly in structure and display elongated and smaller nanostructures15.
Similar data was found with in vivo correlates of iron toxicity which included increases in renal malondialdehyde, renal ferritin, and heme oxygenase-1 expression in mice. These changes also appeared to parallel in vivo glomerular iron uptake (seen withiron sucrose and iron gluconate, but not with iron dextran and iron isomaltoside 1000)28.
I RON ISOMALTOSIDE 1000 AND S-PHOSPHATE
As some parenteral iron therapies have been found to be associated with hypophosphataemia13,26,27,29-32, the effect of iron isomaltoside 1000 on s-phosphate is being measured in several ongoing clinical studies, and interim analyses of s-phosphate data have been performed as part of the protocols. At the current time, these interim analyses included 25 oncology patients and 50 patients with non-dialysis dependent chronic kidney disease (NDD-CKD) treated with iron isomaltoside 1000 (data on file, Pharmacosmos A/S, ClinicalTrial.gov: NCT01145638 (oncology study) and NCT01102413 (NDD-CKD study).
The phosphate analyses are part of two phase III, prospective, open-label, randomised, comparative studies. A total of 350 patients with a diagnosis of non-myeloid cancer and 350 NDD-CDK patients with renal-related anaemia are being randomised 2:1 to either IV iron isomaltoside 1000 (group A) or oral iron sulphate (group B). The patients in group A are equally divided into two sub-groups (A1 and A2). Group A1 are treated with IV iron isomaltoside, where the full iron replacement dose is administered as infusions of maximum 1000 mg iron isomaltoside 1000 over 15 minutes until full replacement dose is achieved. Group A2 are treated with IV bolus injections of 500 mg iron isomaltoside 1000 over 2 minutes, administered once per week until full replacement dose is achieved. Group B are treated with 200 mg oral iron sulphate daily for 8-12 weeks. For the individual patient, the duration of the study is 8-10 weeks in the NDD-CKD study and 24-26 weeks in the oncology study. S-phosphate is measured prior to iron administration and at every visit. At baseline in the oncology study, s-phosphate was 4.0 ± 0.9 mg/dL in group A1 and A2, and 3.6 ± 0.6 mg/dL in group B, and in the NDD-CKD study, s-phosphate was 4.5 ± 0.9 mg/dL in group A1 and A2, and 4.3 ± 0.9 mg/dL in group B.
At interim analysis there was no significant change in the s-phosphate levels in any of the IV treatment groups in either the oncology or NDD-CKD patients. Mean (95 % CI) values of s-phosphate for the three treatment arms are shown in Fig. 3 (oncology study) and Fig. 4 (NDD-CKD study). In other studies, s-phosphate levels below 2 mg/dL have been considered an indicator for hypophosphatemia13,32. In the iron isomaltoside 1000 studies, 3 out of 75 patients experienced a decrease in s-phosphate with a value slightly below 2 mg/dL post treatment. The first patient was a NDD-CKD patient treated with an infusion (1000 mg of iron isomaltoside 1000). The patient had a s-phosphate level of 1.8 mg/dL three weeks after baseline. The second patient was an oncology patient treated with an infusion (also 1000 mg). This patient had a s-phosphate level of 1.9 mg/dL at one and four weeks after baseline. The third patient was an oncology patient treated with two bolus injections (500 mg + 250 mg of iron isomaltoside 1000). The patient already had a low s-phosphate level at the screening visit (2.0 mg/dL), and four weeks after baseline the s-phosphate level was 1.9 mg/dL. All three patients had a s-phosphate which normalized at the following visit. No adverse drug reaction was reported in the three patients.
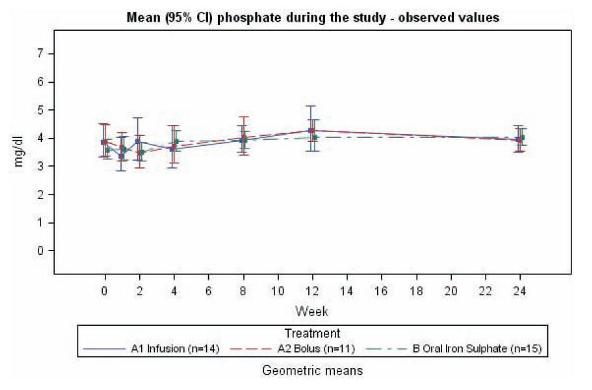
Figure 3
Mean (95 % CI) phosphate values in oncology patients during the 24 week study period. Group A1 was treated with iron isomaltoside 1000 infusion, group A2 was treated with iron isomaltoside 1000 bolus injection, and group B was treated with oral iron sulphate (data on file, Pharmacosmos A/S).
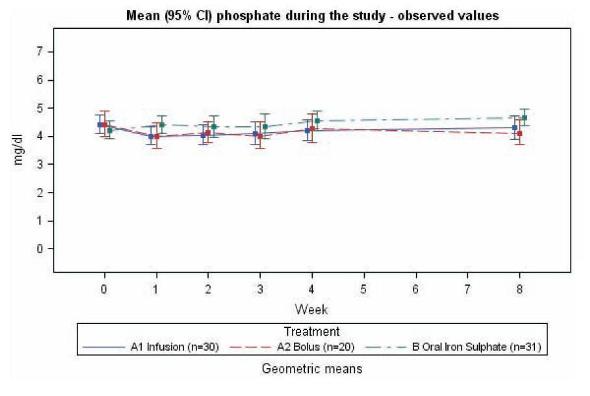
Figure 4
Mean (95 % CI) phosphate values in NDD-CKD patients during the 8 week study period. Group A1 was treated with iron isomaltoside 1000 infusion, group A2 was treated with iron isomaltoside 1000 bolus injection, and group B was treated with oral iron sulphate (data on file, Pharmacosmos A/S).
The interim data suggests that there is unlikely to be clinically significant hypophosphataemic effect associated with iron isomaltoside 1000 treatment.
It has been suggested that the hypophosphataemia associated with parenteral iron therapy could be mediated by Fibroblast Growth Factor 23 (FGF-23)29,33-35; however, the reason for the differences in hypophosphataemic effect observed with the various parenteral iron products still needs to be elucidated and the clinical implications need to be established
COST ANALYSIS OF IRON ISOMALTOSIDE 1000
A cost analysis of iron isomaltoside 1000 and iron carboxymaltose against standard treatments (blood transfusion, iron sucrose, and low molecular weight iron dextran) was performed by comparing the cost of the treatment including nursing costs associated with administration, equipment for administration, and patient transportation in one UK centre36,37. The treatment included three total iron dosage levels, 600 mg, 1000 mg, and 1600 mg for each of the three iron products. This particular analysis indicated that treatment with iron isomaltoside 1000 could provide a net saving when compared with blood transfusion, iron sucrose, and iron carboxymaltose at all three dose levels (Fig. 5). At 600 mg and 1000 mg doses, iron isomaltoside 1000 was also less expensive than low molecular weight iron dextran, but it was more expensive at a dose of 1600 mg. However, low molecular weight iron dextran is administered over six hours, which is inconvenient for the patient and reduces the productivity36,37. These data indicate that iron isomaltoside 1000 can have a cost advantage compared to other parenteral iron products, largely because that high doses may be given with a short administration time and without a test dose.
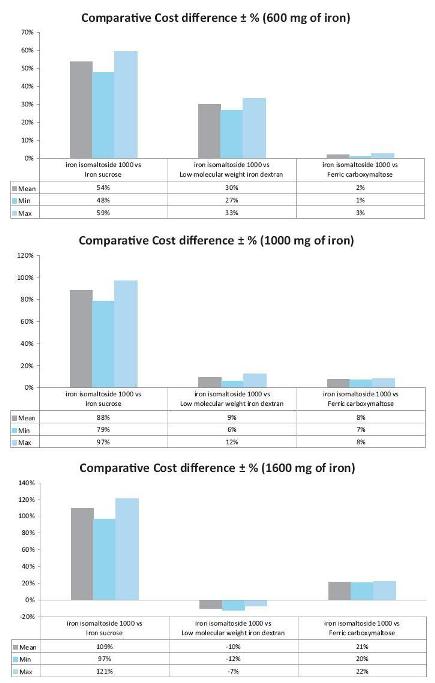
Figure 5
Comparative cost differences (%) for iron isomaltoside 1000 versus iron sucrose, low molecular weight iron dextran, or ferric carboxymaltose administered at three different dose levels (600 mg, 1000 mg, and 1600 mg iron). A positive cost difference indicates a cost saving with iron isomaltoside 1000. The analyses comprised cost of the treatment including nursing costs associated with administration, equipment for administration, and patient transportation. [Modified from Bhandari, 2011 (Ref. 37)].
FURTHER CLINICAL PROGRAMME FOR IRON ISOMALTOSIDE 1000
A substantial additional clinical research programme has been initiated for further exploration of the full clinical and pharmacoeconomic profile of iron isomaltoside 1000. This programme includes ongoing and planned controlled comparative efficacy and safety studies in gastroenterology, nephrology, oncology, gynaecology, surgery, and iron deficiency without anaemia (ClinicalTrial. gov: NCT01145638, NCT01102413, NCT01222884, NCT01017614, NCT01410435, NCT01213979, NCT01280240, NCT01213992, NCT01469078, and NCT01213680). An extensive clinical pharmacology programme applying 100 mg, 200 mg, 500 mg, and 1000 mg doses in selected therapeutic populations is planned. In essence, this extensive programme is tailored for the future as it focuses on safety and convenience of rapid, high-dose monotherapy and pursues administration rates down to 2 minutes for high dose bolus injections and 15 minutes for high dose infusions in ongoing clinical studies.In addition, the programme is applying traditional as well as innovative endpoints with regard to efficacy, and also focuses on pharmacoeconomic benefits.
CONCLUSION
New iron preparations should ideally be capable of delivering a wide dosing range to allow a single visit iron correction dose with no requirement for a test dose, a fast infusion, and minimal potential side effects including low catalytic/labile iron release and negligible risk of anaphylaxis. Furthermore, they should be convenient for the patient and the health care professional, and cost effective for the health care system. The intention behind the development of iron isomaltoside 1000 was to fulfil these demands. This was achieved by depositing the iron at high concentration in a matrix of isomaltoside oligosaccharides, a dextran free homopolymer of glucose units with favourable release properties and low immunogenicity. Iron isomaltoside 1000 has been shown to be effective in treating IDA, and it has a very low immunogenic potential, a very low content of labile and free iron, and does not appear to be associated with hypophosphataemia. Iron isomaltoside 1000 is therefore the only parenteral iron formulation that can be administered as a fast high dose infusion, in doses exceeding 1000 mg, without the need for a test dose. Thus, iron isomaltoside 1000 offers a cost-effective parenteral iron therapy with high dose flexibility, including the possibility of providing full iron correction in one visit thereby optimising the user convenience for the health care professional and the patient.
References
1 McCarthy JT, Regnier CE, Loebertmann CL, Bergstralh EJ. Adverse events in chronic hemodialysis patients receiving intravenous iron dextran–a comparison of two products. Am J Nephrol 2000;20:455-62 [ Links ]
2 Fletes R, Lazarus JM, Gage J, Chertow GM. Suspected iron dextran-related adverse drug events in hemodialysis patients. Am J Kidney Dis 2001;37:743-9 [ Links ]
3 Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant 2004;19:1571-5 [ Links ]
4 Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 2006;21:378-82 [ Links ]
5 Fishbane S. Safety in iron management. Am J Kidney Dis 2003;41(5 Suppl):18-26 [ Links ]
6 Low molecular weight iron dextran (Cosmofer®) summary of product characteristics, dated 27 January 2010
7 Ferrlecit® product monography, dated 6 November 2009
8 Iron sucrose (Venofer®) summary of product characteristics, dated 30 March 2011
9 Ferumoxytol (Feraheme) prescribing information, dated June 2011
10 Agarwal R, Rizkala AR, Kaskas MO, Minasian R, Trout JR. Iron sucrose causes greater proteinuria than ferric gluconate in non-dialysis chronic kidney disease. Kidney Int 2007;72:638-42 [ Links ]
11 Agarwal R, Leehey DJ, Olsen SM, Dahl NV. Proteinuria induced by parenteral iron in chronic kidney disease–a comparative randomized controlled trial. Clin J Am Soc Nephrol 2011;6:114-21 [ Links ]
12 Ferric carboxymaltose (Ferinject®) summary of product characteristics, dated 25 October 2011
13 FDA Advisory Committee Briefing Document, Drug Safety and Risk Management Committee, 1 February 2008. Division of Medical Imaging and Hematology Products and Office of Oncology Drug Products and Office of New Drugs, New Drug Application (NDA) 22-054 for Injectafer (Ferric Carboxymaltose) for the treatment of iron deficiency anemia in patients with heavy uterine bleeding or postpartum patients.
14. Iron Isomaltoside 1000 (Monofer®) summary of product characteristics, dated 26 March 2010
15 Jahn MR, Andreasen HB, Futterer S, Nawroth T, Schunemann V, Kolb U,et al.A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer®), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm 2011;78:480-91 [ Links ]
16 Macdougall IC. Evolution of iv iron compounds over the last century. J Ren Care 2009;35 Suppl 2:8-13 [ Links ]
17 Goldsby R.A., Kindt T.J., Osborn B.A. Immunology. 5th ed. NY: WH Freeman and Co; 2003 [ Links ]
18 Nordfjeld K, Andreasen H, Thomsen LL. Pharmacokinetics of iron isomaltoside 1000 in patients with inflammatory bowel disease. Drug Des Devel Ther 2012;6:43-51 [ Links ]
19 Wikstrom B, Bhandari S, Barany P, et al. Iron isomaltoside 1000: a new intravenous iron for treating iron deficiency in chronic kidney disease. J Nephrol 2011;24:589-96 [ Links ]
20 Hildebrandt PR, Bruun NE, Nielsen OW, et al. Effects of administration of iron isomaltoside 1000 in patients with chronic heart failure. A pilot study. TATM 2010;11:131-7 [ Links ]
21 Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr 1970;100:301-3 [ Links ]
22 Wikstrom B, Bhandari S, Barany P, Kalra PA, Ladefoged S, Wilske J. Monofer, a novel intravenous iron oligosaccharide for treatment of iron deficiency in patients with chronic kidney disease (CKD). Poster No.M560, World Congress of Nephrology, May 22-26 2009, Milan, Italy [ Links ]
23 Wikstrom B, Bhandari S, Barany P, et al. High single dose infusion of iron isomaltoside 1000 (Monofer®) in hemodialysis patients. Poster at the World Congress of Nephrology, 2011 [ Links ]
24 Kalra PA, Bhandari S, Hildebrandt PR, Thomsen LL. High dose infusion of iron isomaltoside 1000 (Monofer®) in CKD and CHF patients. Poster No.Su571 at XLVII ERA-EDTA Congress, Munich, Germany, 25-28 June, 2010 [ Links ]
25 Kalra PA, Bhandari S, Thomsen LL. One visit iron repletion with iron isomaltoside 1000 (Monofer®) in pre-dialysis CKD patients. Poster No.SA-PO 2349, ASN 43rd Annual Meeting, Denver Colorado, November 2010 [ Links ]
26 Sato K, Nohtomi K, Demura H, et al. Saccharated ferric oxide (SFO)-induced osteomalacia: in vitro inhibition by SFO of bone formation and 1,25-dihydroxy-vitamin D production in renal tubules. Bone 1997;21:57-64 [ Links ]
27 Sato K, Shiraki M. Saccharated ferric oxide-induced osteomalacia in Japan: iron-induced osteopathy due to nephropathy. Endocr J 1998;45:431-9 [ Links ]
28 Zager RA, Johnson AC, Hanson SY. Parenteral iron nephrotoxicity: potential mechanisms and consequences. Kidney Int 2004;66:144-56 [ Links ]
29 Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab 2009;94:2332-7 [ Links ]
30 Schouten BJ, Doogue MP, Soule SG, Hunt PJ. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem 2009;46(Pt 2):167-9 [ Links ]
31Okada M, Imamura K, Iida M, Fuchigami T, Omae T. Hypophosphatemia induced by intravenous administration of Saccharated iron oxide. Klin Wochenschr 1983;61:99-102 [ Links ]
32 Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion 2009;49:2719-28 [ Links ]
33 Durham BH, Joseph F, Bailey LM, Fraser WD. The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann Clin Biochem 2007;44(Pt 5):463-6 [ Links ]
34 Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans . J Clin Endocrinol Metab 2011;96:3541-9 [ Links ]
35 Shimizu Y, Tada Y, Yamauchi M, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone 2009;45:814-6 [ Links ]
36 Bhandari S. A hospital-based cost minimization study of the potential financial impact on the UK health care system of introduction of iron isomaltoside 1000. Ther Clin Risk Manag 2011;7:103-13 [ Links ]
37 Bhandari S. Update of a comparative analysis of cost minimization following the introduction of newly available intravenous iron therapies in hospital practice. Ther Clin Risk Manag 2011;7:501-9 [ Links ]
Dr. Philip A. Kalra. Department pf Renal Medicine, Salford Royal Hospital, Salford, United Kingdon E-mail: philip.kalra@srft.nhs.uk
Conflict of interest statement. Professor Philip Kalra has received honoraria for lectures and advisory board participation from Pharmacosmos, Vifor, and Takeda. Professor Klaus Bock has received honoraria for consultancy tasks from Pharmacosmos. Professor Morten Meldal declares that he has no conflicts of any financial interests whatsoever.
Received for publication: 02/03/2012
Accepted: 8/03/2012














