Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Portuguese Journal of Nephrology & Hypertension
versión impresa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.3 Lisboa set. 2013
It's an anti-hyperglycaemic
It's a diuretic It's SGLT2 inhibition!
É um anti-hiperglicémico... É um diurético...
É a inibição SGLT2!
Ines Aires, Joaquim Calado
NOVA Medical School, Departments of Medicine-Nephrology and Genetics
Department of Nephrology, Hospital de Curry Cabral, Centro Hospitalar de Lisboa Central, Lisboa, Portugal.
As we sit down to write this Editorial, the last issue of the Journal of the American Society of Nephrology stands in front of us. Its cover displays an add that publicizes canagliflozin, the first SGLT 2i (Sodium Glucose Transporter 2 inhibitor) approved, earlier this year, by the U.S. Food and Drug Administration (FDA) for the treatment of Type 2 Diabetes Mellitus (T2DM). This follows last year approval by the European Medicines Agency (EMA) of the first compound of its class, dapagliflozin. More than a century has elapsed since the original description of glucosuria induced by phlorizin, the parental compound from which all SGLT 2i got their inspiration. During this period many insights on the homeostasis of sodium and glucose were unravelled. These two solutes are crucial for the survival of all organisms, higher vertebrates in particular, which have developed elaborate pathways to preserve them. However, in face of the high caloric and salt contente of western diets, these phylogenetically conserved pathways have now turned against us and we try desperately to overrun them. They have become therapeutic targets. The story behind SGLT 2 inhibition is not only the story of sodium and glucose conservation by complex organisms, but also, and for the interested nephrologist, on how the proximal tubule supports and integrates the functioning logic of the kidney.
GLUCOSE TRANSPORT
Glucose is an apolar molecule that diffuses poorly across cellular membranes. Two distinct families of glucose transporters are in place to do the job: the ubiquitous GLUT (GLUcose Transporter) transporter family that ensures glucose transport according to its concentration gradient, and the SGLT co-transporter family. The latter class is secondary active in its nature, since it relies on the favourable transmembrane electrochemical gradient for sodium. It is crucial whenever glucose needs to be reabsorbed by epithelial cells from the luminal side of the gastrointestinal tract and renal proximal tubule against its concentration gradient. The SGLTs are encoded by the SLC5 genes, a family that encompasses > 200 members, 13 alone in the human genome. The SGLT1 was cloned, back in 1987, by Ernest Wrights group, at the UCLA1. In fact, most of the seminar work on SGLTs has been fostered by his team, including the recent solving of the crystal structure of SGLT2. We strongly recommend to our readers a recently published review on the subject by this author3 who, in addition, was the recipient of last years ASN Homer W. Smith award.
MOLECULAR GENETICS OF SGLTS ASSOCIATED PHENOTYPES
Mutations in SLC5A1, encoding SGLT 1, are responsible for the Glucose-Galactose Malabsorption syndrome, characterized by watery diarrhoea, upon the introduction of galactose, glucose or lactose in the diet of newborns, in accordance to SGLT 1 major expression in the intestine. Although SGLT 1 has a limited expression in the late (S3 – medullary) proximal tubule, it is SGLT 2 that is predominantly expressed in the kidney and at the early proximal (S1) segment4,5. Therefore, the SGLT 2 coding gene, SLC5A2, has always been considered a natural candidate gene for Familial Renal Glucosuria (FRG), a renal phenotype displaying selective glucosuria in the absence of diabetes. Since the original description of SLC5A2 mutations in FRG, we and others have published and characterized several FRG cohorts (reviewed in 6). The distinct tubular sequential expression and transport kinetics of SGLT2 and SGLT1 act in concert to reabsorb all the glucose from the tubular lumen, so that no glucose will appear in the final urine. The bulk of the filtered glucose is reabsorbed in the early S1 segment by the high capacity but low-affinity SGLT2, while the remaining glucose that escapes SGLT2, will face SGLT1 in the later segments of the proximal tubule that, although having a lower capacity for glucose transport compared to SGLT2, displays a far more greater affinity for the ligand. Finally, the ubiquitous GLUT2 and GLUT1, ensure the efflux of glucose from the baso-lateral side of the epitelial cell into the systemic circulation.
TARGETING URINARY GLUCOSE EXCRETION IN T2DM
In diabetes, there is a paradoxical increase in the renal tubular reabsorption for glucose. Both the maximal transport and the renal threshold for glucose are found to be increased in diabetic individuals7. In addition, T2DM is associated with an increase in renal neoglucogenesis8, particularly in the postprandial state. So, all of these pathways perpetuate hyperglycaemia in diabetes and account for the hypertrophy of the proximal tubule seen in the diabetic kidney. This increase in glucose reabsorption by the hypertrophic diabetic proximal tubule may also interfere with the tubuloglomerular feedback (TGFB) mechanism, by decreasing the amount of solutes (sodium in particular) that is being sensed by the macula densa, derepressing the TGFB and inducing glomerular hyperfiltration, a sine qua non condition for the development of the diabetic nephropathy9.
Inducing urinary glucose excretion (UGE) is, therefore, an appealing and novel strategy for the treatment of T2DM.
SGLT2 INHIBITORS
Phlorizin, a natural product isolated from the root bark of the apple tree, and known since the 19th century to cause glucosuria, has been characterized as a β-Dglucoside (Fig. 1). It is comprised of a single glucose moiety and an aglycone in which two aromatic carbocycles are joined by an alkyl spacer. When phlorizin was subcutaneously administered to partially pancreatectomized rats, the hyperglycaemia was corrected without changing insulin levels, and both insulin sensitivity and insulin secretion were restored to normal10.
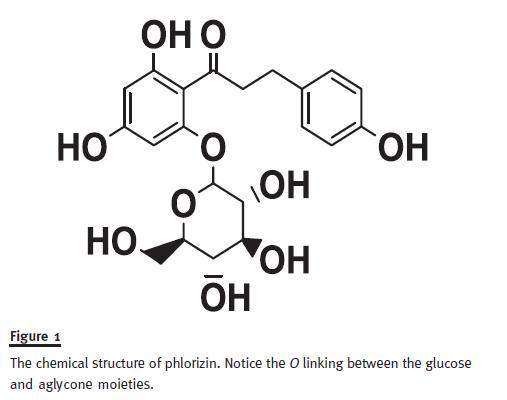
Despite demonstrating utility as an anti-hyperglycaemic agent in several rodent models of diabetes, phlorizin was discontinued. Being an O-glucoside, it was particularly susceptible to intestinal and liver β-glucosidases. In addition, it inhibited SGLT1 equally as well as SGLT2. Furthermore, its active metabolite, phloretin, inhibits non-specifically other glucose transporters.
Structure-activity relationship analysis allowed modification of the original compound, with the development of new C-glucosides resistant to β-glucosidases (enabling oral administration) and subsequent modifications of the aglycone moiety (having higher affinity for SGLT2), leading to the discovery of dapagliflozin11, empagliflozin12 and canagliflozin13, the first three SGLT2i expected to reach the market.
Finally, the finding that FRG, an essentially benign phenotype, could work as a safety model for the chronic pharmacological inhibition of SGLT2, provided the adequate leverage for the final development of these compounds by the pharmaceutical industry.
CLINICAL TRIALS: BENEFITS AND PITFALLS OF SGLT2I
Past the rationale of the pre-clinical development, there is now relevant published data on clinical efficacy and safety profiles for SGLT 2 pharmacological inhibition. Being UGE an insulin independent mechanism, SGLT 2i were found to be useful anti-hyperglycaemic drugs, either alone or in combination with other oral agentes and insulin, not associated with hypoglycaemia. The level of haemoglobin A1c (A1c) reduction is dependente on dosing as well on the pre-treatment level. It ranged from -0.7% to -0.9% (absolute value) in naive patients in monotherapy14 up to -2.6% in T2DM individuals with inadequate glycaemic control having a pre-treatment level of A1c of 10-12%15. It is beyond the scope of this editorial to review the clinical research programme for the several SGLT2i available. Still, a few importante features ought to be highlighted. These compounds are indeed true calorieuretics, in the sense that na UGE of ~70 g/day (the range usually observed in clinical trials) will lead to a caloric loss of 280 kcal. Weight loss is a unique and important characteristic of these compounds, and it can reach 3 kg by the end of the treatment period. An additional observation with SGLT2i, and one that we had already anticipated from our studies on FRG pedigrees, is the diuretic effect16. Urinary osmol losses include both glucose and, most importantly, sodium. This certainly provides the basis for the anti-hypertensive effect observed with SGLT2i and is probably responsible for the good cardiovascular profile reported for this class.
The SGLTi are generally well tolerated. Genital (mycotic) tract infections is one of the most common adverse event (AE) seen with these drugs, affecting ~13% of treated individuals. Usually not severe and easily treated, they should not be a reason for treatment discontinuation.
The UGE is critically, and equally, dependent on both the plasma glucose concentration and the glomerular filtration rate (GFR). As expected, Chronic Kidney Disease (CKD) will lead to a decrease in SGLT2i efficacy. This was ascertained in several clinical trials involving CKD stage 3 (moderate renal impairment) patients, that also reported more AEs compared to placebo, including decreases in estimated GFR17-19. Interestingly, even with CKD, blood pressure control with these compounds was still optimal, reinforcing the idea that they are indeed diuretics.
PENDING ISSUES AND UNSOLVED QUESTIONS
In January 2012, the FDA voted against the approval of dapagliflozin until more data was provided, based on imbalances in small numbers of 2 types of malignancies breast and bladder cancers between dapagliflozin treated and controls20. The Advisory Committee also wrote that Overall, the available data suggest that the likelihood of a causal association between dapagliflozin and bladder cancer is small. This was a surprise for the diabetic community, in desperate need of novel strategies for the treatment of T2DM. Much has been written about the subject, and several reasons for this imbalance have been proposed – from recruitment bias to urine scrutiny (at least for bladder cancer). Some of us argued for the implausibility of the association – not only SGLT 2 is exclusively expressed in the renal proximal tubule (no room for pleiotropy), but also the fact cancers were diagnosed too early in the treatment period (sometimes within the first month). Significantly, these imbalances seen with the first of its class, did not prevent the FDA from approving canagliflozin or EMA, dapagliflozin itself.
More appealing for the nephrologist with a special interest in renal physiology, is the finding of na almost dose dependency reduction in serum uric acid levels seen with SGLT2i, which is surprising in light of the associated diuretic effect of the compound. Also puzzling, are the reported increments in phosphate and parathyroid hormone serum levels, even in non-CKD populations, and the risk of nonosteoporotic fractures in CKD stage 3. Let us not forget that urate (by means of URAT 1 and GLUT9) and phosphate (via NaPi-IIa and NaPi-IIc) are reabsorbed from the luminal side at the proximal tubule, and that 1α hydroxylation of the 25-OH-vitamin D3 also occurs in this nephron segment.
It is a misfortune that so many insights into the function of the proximal tubule are to be gained from a class of compounds nephrologists will seldom use; and then again, it may turn out that we, the nephrology community, may well find novel and ingenious uses for SGLT2 inhibition.
References
1. Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature 1987;330(6146):379–381 [ Links ]
2. Faham S, Watanabe A, Besserer GM, et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 2008;321(5890):810–814 [ Links ]
3. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91(2):733-794 [ Links ]
4. Turner RJ, Moran A. Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol 1982;70(1):37–45 [ Links ]
5. Chen J, Williams S, Ho S, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 2010;1(2):57-92 [ Links ]
6. Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 2010;5(1):133-141 [ Links ]
7. Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest 1971;28(1):101–109 [ Links ]
8. Meyer C, Stumvoll M, Nadkarni V, Doustou J, Mitrakou A, Gerich J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest 1998;102(3):619-624 [ Links ]
9. Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2011;300(5):R1009-1022 [ Links ]
10. Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987;79(5):1510–1515 [ Links ]
11. Meng W, Ellsworth BA, Nirschl AA, et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 2008;51(5):1145–1149 [ Links ]
12. Grempler R, Thomas L, Eckhardt M, et al. In vitro properties and in vivo effect on urinary glucose excretion of BI 10773, a novel selective SGLT2 inhibitor. Diabetes 2009; 58: Suppl 1 Abs 521-P [ Links ]
13. Nomura S, Sakamaki S, Hongu M, et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem 2010;53(17):6355-6360 [ Links ]
14. List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009;32(4):650-657 [ Links ]
15. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217-2224. [ Links ]
16. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: Absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant 2008;23(12):3874–3879 [ Links ]
17. Kohan D, Fioretta P, List J, Tang W. Efficay and safety of dapagliflozin in patients with Type 2 Diabetes and moderate renal impairment. American Society of Nephrology, annual meeting 2011 – November 8-13 Philadelphia (TH-PO524) [ Links ]
18. Bakris GL, Yale JF, Wajs E, Xi L,Usiskin K, Meininger G. Efficacy and Safety of Canagliflozin (CANA) in Subjects with Type 2 Diabetes Mellitus (T2DM) and Moderate Renal Impairment. American Society of Nephrology, 2012 annual meeting – October 30-November 4 San Diego (TH-PO536) [ Links ]
19. Barnett AH, et al. Empagliflozin in Patients with Type 2 Diabetes (T2DM) and Renal Impairment (RI). American Diabetes Association, 2013 annual meeting – June 21-25, Chicago (1104-P) [ Links ]
20. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM262996.Pdf [ Links ]
Professor Joaquim Calado
Department of Nephrology, Hospital de Curry Cabral, Centro Hospitalar de Lisboa Central
Rua da Beneficencia, nº8
1069-166 Lisboa, Portugal
E-mail: jcalado@fcm.unl.pt
Conflict of interest: None declared
Received for publication: 23/07/2013
Accepted: 05/08/2013














