Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Portuguese Journal of Nephrology & Hypertension
versión impresa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.3 Lisboa set. 2013
Evolution of the diagnostic criteria of antibody-mediated rejection of renal allografts: Banff classification updates
Muhammed Mubarak, Javed I. Kazi
Histopathology Department, Sindh Institute of Urology and Transplantation (SIUT). Karachi, Pakistan.
ABSTRACT
Alloimmune rejection is one of the most common causes of both acute and chronic renal allograft dysfunction throughout the world. It is also one of the most important causes of graft loss, both in the short - and the long-term. The process of rejection of the allograft tissue is mediated predominantly by the acquired or specific alloimmune mechanisms of both humoral and T cell-mediated arms. More recently, evidence is also accumulating which supports the role of innate immunity in initiating this process.
The diagnosis of the rejection process may be suspected on clinical grounds, with some support from the laboratory investigations and imaging studies, but the gold standard test for an accurate diagnosis and specifically its categorization is still the invasive procedure of renal allograft biopsy. In addition, numerous molecular markers in the serum and urine have also been investigated as non -invasive alternatives to renal allograft biopsy for the diagnosis of acute rejection, but the renal allograft biopsy provides additional valuable information over and above just the diagnosis of rejection. During early 1990s efforts began to standardize the histopathological study of renal allograft biopsies for the uniform reporting of the pathological lesions. These efforts have continued with a missionary zeal and have resulted in marked refinements in the diagnostic criteria and categories of rejection seen on allograft biopsies. The presente series attempts to address the evolutionary changes in the diagnostic criteria and the classification of the rejection process on renal allograft biopsies as these took place over the years since early 1990s. In this review, we will discuss the changes that have occurred in the diagnosis and categorization of antibody mediated rejection and the focus will be on the morphological basis of their diagnosis and categorization.
Key words: Antibody-mediated rejection; Banff classification; immunofluorescence; kidney; rejection.
The process of alloimmune injury to the graft tissue may be mediated by the cytotoxic T lymphocytes or the antibodies, or more commonly, a variable combination of both, invariably in combination with components of the innate immune system1,2. Traditionally, rejection was divided on clinical grounds into hyperacute, acute, and chronic types, depending on the rapidity of rejection process development with little reference to the underlying pathogenetic mechanisms1. Prior to 1993, there was no single universally accepted system for the diagnosis and categorization of pathological lesions on renal allograft biopsies in general and the rejection in particular3,4. Different transplant centres were developing their own protocols and their own definitions of morphological features5-8.
The Banff schema was formulated as an international, consensus -based classification system for the diagnosis and categorization of renal allograft biopsy pathology with a particular emphasis on the development of the morphological criteria for the diagnosis and classification of rejection9,10. The first Banff conference was held in August 1991 at Banff, Alberta, Canada, and the first product that came out of that meeting was published in 1993 and is popularly known as 1st Banff classification11. Subsequent Banff meetings have taken place every two years, mostly in Canada, but more recently in some other places in the world, to take cognizance of the latest developments in the field of transplant pathology. The majority of the meetings have been followed by updates and revisions in the form of publications in international premier journals of nephrology and transplantation12,13.
There have been substantial changes in both the nomenclature and the classification of rejection reaction, but the basic framework of the Banff schema has remained the same9,12,13. The first major and significant modification of the original Banff classification took place in 1997 with the incorporation and merging of National Institutes of Health (NIH)-sponsored Collaborative Clinical Trials in Transplantation (CCTT) classification and Banff 93-95 classification, the two most widely used classifications at that time14,15. Since then, Banff 97 working classification of renal allograft pathology has served as international yardstick in the field of routine renal transplant pathology practice and the clinical trials13.
As regards the evolutionary changes in the diagnostic category of antibody-mediated rejection (AMR), the first Banff conference dealt with the category in a very superficial and imprecise manner. It was classified and named purely on clinical grounds as hyperacute rejection (HAR)11. The clinical and pathological features of this type of rejection are so dramatic and well characterized that they rarely escape detection (Fig. 1). However, due to almost universal implementation of pretransplant antibody screening and donor-specific cross matching, this form of rejection has almost completely disappeared. It is however apparent from the pathological features of HAR that the main targets of alloantibodies are the microcirculation and the blood vessels rather than the tubules and interstitium, the latter being the prime targets of T-cell mediated rejection. The Banff 97 revised classification also dealt with this category of rejection in an imprecise manner and no attempt was made to define or classify it on the pathological foundations.
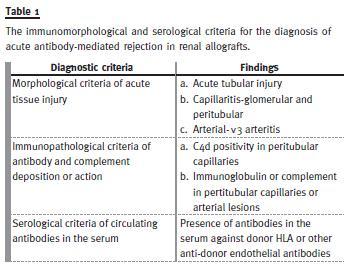
The Banff 97 classification, however, identified two types of clinical presentation of the AMR and classified it into HAR and delayed (accelerated acute) forms14. The Banff 2001 classification was the first to address the issue of AMR in sufficient detail and formulated the morphological, immunopathological and serological criteria for the conclusive diagnosis of AMR16. In fact, the title of the report of Banff 2001 meeting, which was published in 2003, was reflective of this evolution. This evolution was the direct result of an exponential rise in the number of studies on AMR diagnosis in general and the discovery and widespread use of a relatively sensitive and specific marker of AMR, i.e. complement fragment of 4d (C4d)17 -28. The Banff criteria for the diagnosis of AMR are shown in Table 1 and, as alluded to previously, consist not only of the morphological changes of acute allograft injury, but also the immunopathological evidence (C4d or immunoglobulin positivity along peritubular capillary walls) and the serological evidence in the form of circulating donor -specific antibodies (DSA). The morphological features of AMR were also dealt with in detail in this updated classification and a pathological classification of AMR was formulated16. This represented the first pathology based classification of the process of AMR (Table 2). However, only a single category of AMR was recognized in the Banff 97 update classification. In 2005 Banff classification, the diagnostic category of AMR was further subdivided into 2 types: acute AMR and chronic active AMR29. During subsequent Banff meetings, the criteria for C4d positivity and its scoring and the semiquantitative scoring of the inflammatory lesions of peritubular capillaritis (ptc) have also been developed, validated and incorporated into the classification30-32. With continued expansion of literature on donor specific antibodies, it has been realized that not all antibodies present before, at or after transplantation are associated with alloimmune injury and not all antibody-mediated phenotypes inevitably lead to accelerated allograft loss. Hence, in Banff 07 classification, the term of AMR was replaced by antibody-mediated changes30. The morphological features of chronic active AMR were also listed as glomerular double contours and/or peritubular capillary basement membrane multilayering and/or interstitial fibrosis/tubular atrophy (IFTA) and/or fibrous intimal thickening of arteries (Fig. 2). As is evident, the later two features are not specific for chronic active AMR and require fulfilment of all three criteria for definitive diagnosis of AMR. As a result, the category of AMR is now sufficiently characterized to allow its accurate diagnosis and management in the majority of cases. It must be pointed out here that the changes of AMR may coexist and be obscured by borderline changes, acute or chronic active T-cell-mediated cellular rejection, interstitial fibrosis/tubular atrophy and other category of the Banff classification and none of the morphological features of AMR is completely specific31. Table 3 depicts the key changes that have taken place over the years in the category of AMR in Banff revisions.

With the widespread use of C4d, it has also become apparent that there are some cases which are positive for C4d without any morphological evidence of graft injury on the biopsy. For this, a category of C4d deposition without morphological evidence of active rejection was added in Banff 07 classification under the category of antibody-mediated changes30. The incidence of this finding is reported as high as 25-80% on protocol biopsies in patients with ABO incompatible grafts. Only 4-12% of these patients develop features of acute AMR. Therefore, at this time the significance of this category is still largely unknown. In the 11th Banff meeting, a major outcome was the acknowledgement of the existence of the category of C4d negative AMR in kidney transplants32. It was also recognized that AMR is associated with heterogeneous phenotypes even within the same type of transplanted organ. It was felt that further refinement of the diagnostic criteria of AMR, especially for the category of C4d negative AMR, is needed and for this purpose a new working group was created to define the diagnostic criteria for AMR in kidneys independent of C4d. The results of this working group are expected to be presented at the 12th Banff meeting to be held in Brazil in 2013. Till that time, no changes were made in the diagnostic criteria of AMR from the earlier updates.
In conclusion, major and significant changes have occurred in the diagnostic category of AMR in the Banff classification of renal allograft pathology over the last two decades. This predominantly became possible with the discovery and widespread use of C4d antibody and the use of highly sensitive assays for the detection and characterization of DSA. However, much work remains to be done to fully define the phenotype of AMR in kidney and other solid organ transplants and Banff working groups are actively seeking the evidence -based criteria for fuller characterization of all phenotypes of AMR.
References
1. Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med 2010;363(15):1451 -1462 [ Links ]
2. Ponticelli C. The mechanisms of acute transplant rejection revisited. J Nephrol 2012; 25(2):150 -158 [ Links ]
3. Waiser J, Schreiber M, Budde Ket al.Prognostic value of the Banff classification. Transpl Int 2000;13 Suppl 1:S106 -111 [ Links ]
4. Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine–associated chronic nephropathy. N Engl J Med 1984;311(11):699 -705 [ Links ]
5. Mihatsch MJ, Thiel G, Basler V, et al. Morphological patterns in cyclosporine-treated renal transplant recipients. Transplant Proc 1985;17(4 Suppl 1):101 -116 [ Links ]
6. Pascual M, Vallhonrat H, Cosimi AB, et al. The clinical usefulness of the renal allograft biopsy in the cyclosporine era: a prospective study. Transplantation 1999; 67(5):737 -741 [ Links ]
7. Bergstrand A, Bohman SO, Farnsworth A. Renal histopathology in kidney transplant recipients immunosuppressed with cyclosporine A: results of an international workshop. Clin Nephrol 1985;24(3):107 -119 [ Links ]
8. Farnsworth A, Hall BM, Ng ABP, et al. Renal biopsy morphology in renal transplantation. A comparative study of the light -microscopic appearances of biopsies from patients treated with cyclosporin A or azathioprine prednisone and antilymphocyte globulin. Am J Surg Pathol 1984;8(4):243-252 [ Links ]
9. Solez K. History of the Banff classification of allograft pathology as it approaches its 20th year. Curr Opin Organ Transplant. 2010;15(1):49-51 [ Links ]
10. Colvin RB. The renal allograft biopsy. Kidney Int 1996;50(3):1069-1082 [ Links ]
11. Solez K, Axelsen RA, Benediktsson H, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 1993;44(2):411-422 [ Links ]
12. Weening JJ. The art of classifying renal allograft pathology. Nat Clin Pract Nephrol 2008;4(8):420-421 [ Links ]
13. Solez K, Racusen LC. The Banff classification revisited. Kidney Int 2013;83(2):201 -206. [ Links ]
14. Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999;55(2):713 -723 [ Links ]
15. Colvin RB, Cohen AH, Saiontz C, et al.Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. J Am Soc Nephrol 1997;8(12):1930-1941 [ Links ]
16. Racusen LC, Colvin RB, Solez K, et al. Antibody -mediated rejection criteria –na addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 2003;3(6):708 -714 [ Links ]
17. Feucht HE, Felber E, Gokel MJ, et al. Vascular deposition of complement –split products in kidney allografts with cell -mediated rejection. Clin Exp Immunol 1991;86:464-470 [ Links ]
18. Feucht HE, Schneeberger H, Hillebrand G, et al. Capillary deposition of C4d complemente fragment and early renal graft loss. Kidney Int 1993;43(6):1333-1338 [ Links ]
19. Collins AB, Schneeberger EE, Pascual MA, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol 1999;10(10):2208-2214 [ Links ]
20. Mauiyyedi S, Pelle PD, Saidman S, et al. Chronic humoral rejection: identification of antibody -mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol 2001;12(3):574-582 [ Links ]
21. Bohmig GA, Exner M, Watschinger B, et al. C4d deposits in renal allografts are associated with inferior graft outcome. Transplant Proc 2001;33(1 -2):1151-1152 [ Links ]
22. Bohmig GA, Exner M, Habicht A, et al. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol 2002;13(4):1091-1099. [ Links ]
23. Mauiyyedi S, Crespo M, Collins AB, et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol 2002;13(3):779-787 [ Links ]
24. Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ. Detection of thecomplement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol 2002;13(1):242-251 [ Links ]
25. Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long -term prognostic factor. J Am Soc Nephrol 2002;13(1):234 -241 [ Links ]
26. Mubarak M. Renal allograft pathology with C4d immunostaining in patients with graft dysfunction. Indian J Nephrol 2012;22(3):233-234 [ Links ]
27. Kazi J, Mubarak M. Biopsy findings in renal allograft dysfunction in a live related renal transplant program. J Transplant Tech Res 2012; 2:108 [ Links ]
28. Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant 2004;4(10):1562-1566 [ Links ]
29. Solez K, Colvin RB, Racusen LC, et al. Banff 05 meeting report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (CAN). Am J Transplant 2007;7(3):518-526 [ Links ]
30. Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant, 2008;8(4):753-760 [ Links ]
31. Sis B, Mengel M, Haas M, et al. Banff 09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 2010;10(3):464 -471 [ Links ]
32. Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant 2012;12(3):563-570 [ Links ]
Prof. Dr. Muhammed Mubarak
Histopathology Department,
Sindh Institute of Urology and Transplantation.
Karachi - 74200, Pakistan,
E-mail: drmubaraksiut@yahoo.com
Conflicts of interest statement: None declared.
Received for publication: 11/07/2013
Accepted: 01/08/2013














