Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.4 Lisboa dez. 2013
ORIGINAL ARTICLE
Outcomes assessment with two different medication administration modalities for the treatment of secondary hyperparathyroidism
Avaliação comparativa de duas modalidades de administração de medicação para o tratamento do hiperparatiroidismo secundário
Jose Reimao Pinto, Pedro Ponce
Nephrocare, Restelo and Lumiar. Lisbon, Portugal.
ABSTRACT
Background: The treatment of hyperparathyroidism in dialysis patients relies on adequate control of serum phosphorus (Pi) and PTH levels. Patient (pt) compliance to oral therapy at home is known to be quite poor and cannot be monitored. There is evidence that pulse (every other day) therapy with vitamin D compounds is as effective as daily administration. We hypothesized that the same might apply to calcimimetics. Objective: To assess the effect on metabolic endpoints of changing cinacalcet oral treatment from a daily, home-based administration (H), to a three-times/week witnessed administration at the end of each dialysis session in the clinic (C). Patient and methods: 93 prevalent dialysis pts in 6 clinics were included in an observational retrospective study, each patient serving as his own historical control. Inclusion criteria were: ESRD treated by haemodialysis; PTH levels > 500 ng/L and cinacalcet prescription for more than 9 months. Data was drawn from a database common to all participating clinics – EuCliDc. Blood samples were collected at the beginning of dialysis and treated at a central laboratory. Calcium (Ca), phosphorus, albumin and PTH were registered every 3 months, as well as vitamin D and cinacalcet dosages, in the last 9 months of period H and first 9 months of period C. Values during H prescription were compared with values after change to C administration through paired samples t-test. Results: We detected a significant reduction in the dose of Cinacalcet from an average dose of 1221mg/month in period H to an average dose of 674.4mg/month in period C, p < 0.0001 (95% CI 689.4 / 405.1), keeping identical dosages of Vitamin D metabolite 21.3 mic/month vs. 31.9mic/month, p=.167. This dose reduction was translated, as expected, in cost savings, going from an average Cinacalcet cost of 256/month/pt to 137.6/month/patient, p < 0.0001. PTH was more elevated in the C group 903.5 vs. 807.9ng/L, < 0.028, phosphate levels were identical, 5.0 in period H and 4.8mg/dl in period C, p = 0.065. Conclusion: Administration of Cinacalcet under supervision 3 times per week, post -dialysis, was shown to be safe, guarantees compliance and saved considerable resources for the same therapeutic efficacy.
Key Words: Cinacalcet; compliance; haemodialysis; hyperparathyroidism.
RESUMO
Contexto: O controlo do hiperparatiroidismo nos doentes em diálise, passa pela adequação dos níveis do Fósforo e da PTH. A aderência dos doentes à terapêutica oral no domicílio é reconhecidamente deficiente e impossível de monitorizar. Estando estabelecida a evidência que terapêutica em pulsus orais (em dias alternados) com metabolitos activos da vitamina D é tão eficaz como a terapêutica diária, quisemos testar se o mesmo se aplicava à terapêutica com Cinacalcet. Objectivo: Avaliar o efeito no metabolism ósseo da mudança da terapia com Cinacalcet, de um regime diário no domicílio (H), para a administração supervisionada na clínica (C) 3 vezes por semana, no final de cada diálise. Doentes e Métodos: 93 doentes prevalentes em tratamento de hemodiálise em 6 clínicas foram incluídos neste estudo observacional, retrospectivo, cada doente servindo como seu próprio controlo histórico. Os critérios de inclusão exigiam valores de PTH > 500ng/L e tratamento com Cinacalcet há mais de 9 meses. A informação foi colhida de uma base de dados comum a todas as clínicas envolvidas – EuCliD©. As amostras de sangue foram colhidas no início de cada tratamento e analisadas em laboratório central. Os valores de cálcio, fósforo, albumina e PTH foram registados trimestralmente, bem como as doses de terapêutica com metabolitos da vitamina D e Cinacalcet, nos últimos 9 meses do período H e primeiros 9 meses do período C. Os valores durante a prescrição H foram comparados com os valores obtidos após a mudança para a prescrição C através de test-t emparelhado. Resultados: Verificámos existir uma acentuada redução na dose de Cinacalcet de um valor médio de 1221mg/ mês no período H para um valor médio de 674.4mg/mês no período C, p<0.0001 (95% CI 689.4 / 405.1), mantendo doses idênticas de vitamina D (Paricalcitol) de 21.3 mic/mês vs 31.9mic/mês, p=.167. Esta redução de dose teve uma tradução esperada de redução de custos de um custo médio de Cinacalcet de 256/mês/doente para 137.6/mês /doente, p< 0.0001. O valor de PTH foi mais elevado no grupo C 903.5 vs 807.9ng/L, p < 0.028, os níveis de fósforo foram idênticos, 5.0 no período H e 4.8mg/dl no período C, p=0.065. Conclusão: A administração supervisionada de Cinacalcet 3 vezes por semana, mostrou ser segura, garantir a aderência terapêutica e economizar consideravelmente para a mesma eficácia terapêutica.
Palavras Chave: Aderência terapêutica; cinacalcet; hemodiálise; hiperparatiroidismo.
INTRODUCTION
The treatment of hyperparathyroidism in dialysis patients relies on adequate control of serum phosphorus (Pi) and PTH levels. Patient compliance to oral therapy at home is known to be quite poor and cannot be monitored.
In our current reimbursement system, the comprehensive price, for the treatment of end-stage renal failure with outpatient haemodialysis, the cost of drug treatment for bone and mineral disorders in CKD are totally included in the bundle supported by the provider. Controlling all aspects of care, it became apparent that we had an enormous waste of extremely expensive medication that was distributed but not taken at home by our patients, probably with a negative impact in their care and outcomes.
There is evidence that pulse (every other day) therapy with vitamin D compounds is as effective as daily administration. We hypothesized that the same might apply to calcimimetics.
This study was designed to assess the effect on metabolic endpoints, compliance and medication costs of changing cinacalcet oral treatment from a daily, home -based administration (H), to a three-times/week witnessed administration at the end of each dialysis session in the clinic (C).
PATIENT AND METHODS
This was a retrospective, observational study, using data from Nephrocare permanent registry EuCliDR (European Clinical Database).
Data from seven dialysis units in our network that have changed, at least nine months before data extraction, their Cinacalcet prescription from a daily dosage to three times per week at the end of the dialysis treatment.
In each one of these clinics, all prevalent patients with secondary hyperparathyroidism, being treated with cinacalcet at the discretion of their attending nephrologist were included, each patient serving as his own historical control. Inclusion criteria were: ESRD treated by haemodialysis; PTH levels > 500 ng/L and cinacalcet prescription for more than 9 months.
Patient data was analysed every 3 months since 9 months prior to the change in the administration modality and for 9 months after that change.
Data was collected every 3 month for iPTH, calcium, phosphorus, Cinacalcet dose and Vitamin D dose in that same month, allowing a wash out period of 3 months before the first laboratory assessment after the modality change from H to C: Periods T-9, T-6, T-3, T+3, T+6, T+9.
Blood samples were collected in all units at the beginning of dialysis and treated at a central laboratory.
PTH was measured by ECLIA – COBAS 8000 technique from RocheR (reference value 15 to 65ng/L, VC 3.4% – 23.2ng/L).
Laboratory values, as well as medication consumption and its costs during the at home (H) prescription, were compared with values after change to clinic (C) administration through the t -test for paired samples using SPSS software. A Pearson correlation was determined between the cinacalcet dosage and PTH levels at all points in time.
RESULTS
Out of a population of 940 patients, 93 fulfilled the inclusion criteria with complete data to be compared among the 2 periods H and C.
We detected a significant reduction in the dose of Cinacalcet from an average dose of 1221mg/month in period H to an average dose of 674.4mg/month in period C, p < 0.0001 (95% CI 689.4 / 405.1) (Fig. 1), keeping identical dosages of Vitamin D metabolite 21.3 mic/month vs, 31.9mic/month, p =.167.
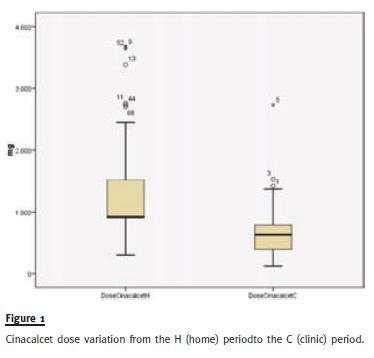
This dose reduction was translated, as expected, in cost savings, going from an average Cinacalcet cost of 256/month/pt to 137.6/month/patient, p < 0.0001 (95% CI 145.6 / 91.1), a reduction on average of 47%.
PTH was more elevated in the C group 903.5 vs. 807.9ng/L, p < 0.028 (Fig. 2), as well as serum calcium 8.7 vs. 8.6mg/dl, p = 0.024, phosphates were identical, 5.0mg/dl in period H and 4.8mg/dl in period C, p =0.065.
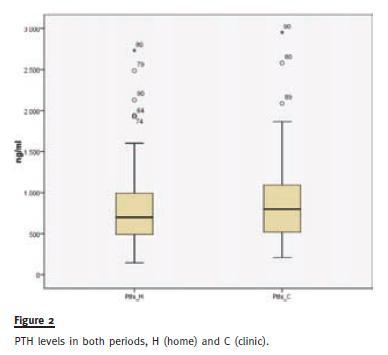
DISCUSSION
Extra -dialytic medication costs on average 25 per dialysis treatment, but unfortunately such a substantial slice of the total treatment cost of end-stage renal failure, even when distributed for free to our patients, does not always achieve the expected clinical effect because, due to several reasons, quite frequently is not regularly, or at all taken.
An increased risk of complications, hospitalization and death associated with dialysis non–compliance is well documented for haemodialysis patients. Non-compliance rates of 50% or higher have been repeatedly reported for varying aspects of the haemodialysis treatment regimen (1 -3).
In our network, we witnessed all too frequently examples of patients that receive their medication monthly, according to what is prescribed, but, although being aware of its importance for their health maintenance and its cost, never take it. Trying to fight this trend, some of us changed the prescription of Cinacalcet, one of the most expensive medications administered to our patients, from a once daily pill taken at home, for a 3 times per week regimen at the end of dialysis, under staff supervision.
Cinacalcet half -life is around 40 hours, 90% found in circulation protein -bound. These pharmacokinetic facts justified our trial.
We demonstrated that with significantly lower doses of Cinacalcet, obtaining quite relevant savings, we were able to achieve an identical control of hyperparathyroidism with identical phosphate levels, without extra dosage of Vitamin D metabolites.
We looked at a heterogeneous population of patients, some were probably compliant during the period of home administration, others were not.
These two types of patients will have dramatic different behaviours with these two modalities of medication administration and, unfortunately, we cannot identify who were the compliant patients in that first study period.
We could not find a correlation between the dosages of Cinacalcet and PTH values. We would expect a negative correlation in period C if indeed the compliance had improved substantially in the clinic period. However, these two variables are not independent and it is more probable that Cinacalcet prescribed dose increased in response to higher PTH values, obscuring the negative correlation we were looking for.
Compliance was per definition 100% in the clinic period, as medication was administered under direct staff supervision, unfortunately we were not able to quantify non -compliance in the first H period. Non-compliance assessment and prevention in chronic disease has indeed demonstrated to be a daunting task, so far never solved with success at least in the dialysis field.
Apparently, every other day Cinacalcet prescription appeared to be safe and not inferior to achieve our main goal of hyperparathyroidism control. As a matter of fact, there is a suggestion that this form of pulse therapy inducing fluctuations in PTH levels control, could prevent some adverse effects in bone metabolism attributed to excessive PTH suppression (personal communication).
Most of us, when changing the prescription schedule, kept the same daily dose but given every other day, three times per week (halving the weekly dose of Cinacalcet), making in the following months upgrade adjustments whenever it was felt that our metabolic endpoints were not reached.
The main limitation of this study is its retrospective analysis. The fact that the dramatic decrease of cinacalcet dose administered in the clinic was associated with only a slight increase in PTH levels, is suggestive of gross non-compliance during the previous home period. A randomized prospective trial is necessary to confirm the efficacy of intermittent supervised cinacalcet administration to control PTH levels.
Compliance can only be improved with better education leading to increase patient autonomy and responsibility, as mostly all other strategies to boost compliance failed so far. Meanwhile, administration of Cinacalcet, as well as other traditionally once daily medication, such as B vitamins, folic acid, vitamin D metabolites , 3 times per week at the end of dialysis, under strict staff supervision by -passes this compliance problem, avoids an enormous waste of money and, hopefully, will achieve better outcomes.
References
1. Leggat JE, Orzol SM, Hulbert -Shearon TE, et al. Noncompliance in hemodialysis: predictors and survival analysis. Am J Kidney Dis 1998;32(1):139 -145 [ Links ]
2. Blever AJ, Hylander B, Sudo H, et al. An international study of patient compliance with hemodialysis. JAMA 1999;281(13):1211 -1213 [ Links ]
3. Kutner NG, Zhang R, McClellan WM, Cole SA. Psychosocial predictors of non -compliance in haemodialysis and peritoneal dialysis patients. Nephrol Dial Transplant 2002;17(1):93-99. [ Links ]
Dr. Pedro Ponce
NephroCare Portugal
Rua Prof. Salazar de Sousa, Lote 12
1750 -233 Lisboa, Portugal
E-mail: Pedro.ponce@fmc-ag.com
Conflict of interest: Both authors work with Nephrocare / Fresenius Medical Care, the protocol design is their only responsibility, they did not receive any industry sponsorship or researcher fees.
Acknowledgements: All the statistical analysis was performed by Pedro Goncalves from SIN (Sistemas Informaticos Nephrocare). We thank our colleagues: Anibal Ferreira, Augusta Gaspar, Carlos Pires, Ilidio Rodrigues, Joao Cruz, Jose Galvao and Pedro Neves, medical directors of the dialysis units surveyed in this study.
Received for publication: 29/07/2013
Accepted in revised form: 05/11/2013














