Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Portuguese Journal of Nephrology & Hypertension
versión impresa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.1 Lisboa mar. 2014
EDITORIAL
Evolution of the approaches toward grading and classifying chronic changes in the renal allograft: Banff classification updates III
Muhammed Mubarak, Javed I. Kazi
Histopathology Department, Sindh Institute of Urology and Transplantation (SIUT). Karachi, Pakistan
ABSTRACT
Currently, the most challenging problem in the field of renal and other solid organ transplantation is the development of chronic progressive sclerosing changes in the allograft. These occur almost uniformly in all renal allografts at a rate of 2-4% per year. In addition, such changes are also quite prevalent in well functioning grafts, as revealed by protocol biopsies. The chronic changes involve all the four components of the renal graft parenchyma, i.e., the glomeruli, blood vessels, tubules and interstitium. Among these, the glomerular and vascular changes are helpful in defining the causes of chronic changes, especially chronic rejection, but are more prone to sampling error, notably the blood vessels, whereas the tubulo-interstitial changes are less specific. However, because the later are less prone to sampling error, these are used for grading the severity of chronic changes in the Banff formulation. The identification, classification and grading of not only the acute but also the chronic changes is of vital importance in guiding the management and predicting the long-term graft outcome of renal transplant recipients. Banff has addressed the issue of chronic changes in detail in its first formulation, as well as its subsequent modifications. However, the magnitude of changes in this category that have occurred over the last two decades is less than that observed in the categories of antibody-mediated and T-cell-mediated rejections. This review describes in detail the changes that have taken place in the category of chronic allograft damage, as the original Banff classification has undergone updates regularly in the last two decades.
Key-words: Banff schema; chronic allograft nephropathy; chronic rejection; interstitial fibrosis; tubular atrophy.
Currently, the most formidable challenge in the field of renal transplantation is the development of chronic, progressive, sclerosing changes in the allograft1,2. These occur almost uniformly in all renal allografts at a rate of 2-4% per year3. In addition, the chronic parenchymal changes are also quite prevalent in well functioning grafts, as revealed by protocol biopsies4. The chronic changes usually involve all the four components of the renal graft parenchyma, i.e., the glomeruli, blood vessels, tubules and interstitium, in variable proportions and combinations3,4. Among these, the glomerular and vascular changes are helpful in defining the causes of chronic changes, especially chronic rejection, but are more prone to sampling error, especially the blood vessels, whereas the tubulo-interstitial changes are less specific. However, because the later are less prone to sampling error, these are used for grading the severity of chronic changes in the Banff formulation.
The identification, classification and grading of severity of not only the acute, but also the chronic changes, is of vital importance in guiding the management and predicting the long-term graft outcome of renal transplant recipients5-7.
As already described in our previous reviews on the Banff classification updates, prior to 1991, there was no single internationally standardized system for the uniform reporting of the pathological lesions on renal allograft biopsies8-12. The Banff working classification represented the first step towards formulation of an international, consensus-based and structured classification system for the diagnosis and categorization of renal allograft biopsy pathology10-12.
To realize this objective, the first Banff meeting was held at Banff, Alberta, Canada on August 2-4, 1991 and the first detailed report that narrated the outcome of the meeting was published in 1993 and is widely known as Banff 93 classification13. This was then followed by regular, follow-up meetings every two years, mostly in Banff, Canada, but more recently, also in some other places of the world, to continuously update and adapt the classification to the latest developments and advances in the field of renal transplant pathology14-21. Almost all the meetings have been followed by updates and revisions in the original Banff schema with the modifications highlighted in papers published in the premier journalsof nephrology and transplantation14-21. The Banff schema not only addressed the issue of acute allograft pathology, but also dealt with the chronic fibrosing changes in sufficient detail. Although the basic framework of the schema and the semiquantitative lesion scoring have largely remained the same, there have occurred significant changes in both the nomenclature and the classification of the original diagnostic categories including category 5 which dealt with the chronic fibrosing changes in the allograft11,12,14-20.
The history of evolution of the Banff classification concerning the terminology and classification of chronic fibrosing changes in the allograft is interesting and reflects our continued and better understanding of the pathophysiology of these changes. It is important to clarify at the outset that the terms acute and chronic in the context of transplant pathology do not always equate with their usual pathological connotations13. In this regard, it is pertinent to note here that some chronic changes including fibrous intimal thickening and tubular atrophy may be present in renal allograft biopsies at the time of transplantation. These constitute the so-called donor-related changes. The time-zero (implantation) biopsies are essential to document such changes, so that they serve as useful reference for the interpretation of future renal allograft biopsy lesions.
Prior to 1991, the term chronic rejection was widely used for all causes of chronic allograft dysfunction17.
This was unfortunate, in that the chronic changes in the allograft are not only caused by alloimmune mechanisms but also by the non-immune mechanisms. In fact, these non-immune mechanisms may be more prevalent than the alloimmune mechanisms in many cases. The Banff formulation introduced the term chronic allograft nephropathy (CAN), in 1991, as a more generic alternative to the misleading and the then popular term of chronic rejection13. The Banff 93 classification divided CAN into three grades based on the extent of tubular atrophy and interstitial fibrosis. No subdivision of CAN category was made and all causes of chronic changes were lumped together in this one category13. This again was unfortunate in that this discouraged or rather prompted the transplant pathologists to render the diagnosis of CAN with little effort to look for specific features of chronic diseases affecting the graft. In addition, many morphological features of, for instance, chronic antibody-mediated rejection, were not known at that time. It is also apparent from a close scrutiny of Banff 93 schema that the main focus of this classification was on the diagnosis and categorization of acute/active cell-mediated rejection13. There is very little discussion on the diagnosis or categorization of chronic changes in this classification.
In the Banff 1995 report, chronic allograft nephropathy index (CADI) was integrated with the CAN category to grade the severity of the chronic changes14.
No subclassification of CAN was, however, made in this update of the original classification.
In the Banff 97 classification, an attempt was made toward encouraging the pathologists to seek closely the specific features of chronic changes in the allograft, and identify and document these changes (Table 1). Some of these specific morphological features are shown in Figures 1 through 4.
Fig. 2
Fig. 3
A subdivision of each of the grades of rejection into a and b category was done depending on the absence or presence of specific features related to chronic rejection15. The grading of the CAN category however remained the same as in previous classifications. No changes in the nomenclature or grading of CAN were made in 97-update classification (Banff 2001 meeting) or Banff 2003 meeting reports.
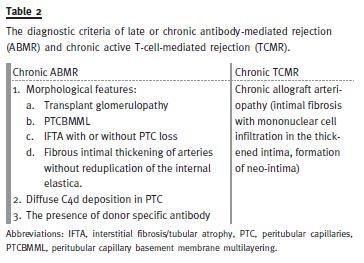
In the meantime, although the advent of CAN terminology implied that it is a non-specific and noncommittal term just describing the chronic fibrosing changes affecting the graft, the widespread use of this term, in turn, resulted in the misunderstanding that this is a specific disease entity. The term remained in use for a good period of 14 years before it was eliminated in 2005 Banff meeting. A major change in the category of chronic fibrosing changes thus occurred in the Banff 2005 meeting with the result that the term CAN was eliminated and replaced by interstitial fibrosis/tubular atrophy (IFTA), no evidence of specific aetiology18. The causes of a subcategory of CAN in previous classifications were moved to the other category, while the chronic alloimmune causes were included in the respective categories of antibodymediated rejection (ABMR) and T-cell-mediated rejection (TCMR) as chronic active types (Table 2). Thus, category 5 in the Banff 2005 and all subsequent updates, now includes only those cases of chronic changes for which no specific aetiological features can be found on the biopsy (Table 3).
In the Banff 2009 meeting, a new development took place in the form of establishment of Banff Working Groups (BWGs) to address the problematic areas of the Banff classification in more detail20. A working group was also established to assess the problems of the definition, interpretation and quantification of fibrosing injury in the renal allografts and native kidneys.
Preliminary results by the BWG have shown a good correlation between the visual fibrosis scoring using the trichrome stain with the computer and immunohistochemical (IHC) stains based image analysis methods. The group is further refining the diagnostic criteria and testing various staining methods and techniques to further improve the interobserver reproducibility and the predictive value of the chronic fibrosing changes for the ultimate graft outcome20.
A number of additional approaches have also been investigated to better quantify the degree of chronic changes in the renal allograft. These include among others computer based image analysis after Sirius Red staining, computerized morphometry combined with IHC stains with type III collagen, smooth muscle actin and tenascin, and the quantification of mast cells as a surrogate marker for the allograft fibrosis22. However, almost all morphometric methods are time consuming, tedious and have intrinsic limitations related to sampling error. The improvements in scanning, spectral analysis, and the computer software in the future will provide better measurements of fibrosis for the objective and standardized evaluation of renal allograft biopsies and the outcome analysis of therapeutic trials for renal fibrosis. The prognostic value of the quantitative image evaluation might also be increased by techniques addressing the dynamics of fibrosis matrix generation.
The molecular basis of renal allograft fibrosis is complex and intimately interrelated with the primary processes leading to the renal allograft injury. A complete understanding of the molecular pathways in future can lead to the discovery of targeted therapies aimed at arresting fibrosis at early stage precluding the irreversible scarring that may follow.
It is worth emphasizing here that the category of chronic sclerosing changes can co-exist with any of the other categories of renal allograft pathology, except 1, which is normal. It is important to document both the acute and chronic pathological lesions on the biopsies to guide the treatment and inform the prognosis.
In conclusion, the categorization and classification of chronic changes have undergone significant changes during the last two decades of the Banff consensus process. More recently, efforts have been made to identify the specific features relevant to the causes of chronic allograft damage. This has been facilitated by the ancillary techniques of immunohistochemistry and electron microscopy. In future, molecular profiling may help pinpoint the causes of chronic allograft damage and this will ultimately help in the optimal long-term graft outcome.
References
1. Nankivell BJ, Chapman JR. Chronic allograft nephropathy: current concepts and future directions. Transplantation 2006;81(5):643-654. [ Links ]
2. Nankivell BJ, Borrows RJ, Fung CL, OConnell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med 2003;349(24):2326-2333. [ Links ]
3. John R, Herzenberg AM. Our approach to a renal transplant biopsy. J Clin Pathol 2010;63(1):26-37. [ Links ]
4. Legendre C, Thervet E, Skhiri H, et al. Histologic features of chronic allograft nephropathy revealed by protocol biopsies in kidney transplant recipients. Transplantation 1998;65(11):1506-1509. [ Links ]
5. Gaber LW. Role of renal allograft biopsy in multicentre clinical trials in transplantation. Am J Kidney Dis 1998;31(6 Suppl 1):S19–S25. [ Links ]
6. Pascual M, Vallhonrat H, Cosimi AB, et al. The clinical usefulness of the renal allograft biopsy in the cyclosporine era: a prospective study. Transplantation 1999;67(5):737-741. [ Links ]
7. Serón D, Anaya F, Marcén R, et al. Guidelines for indicating, obtaining, processing, and evaluating kidney biopsies. Nefrologia 2008;28(4):385-396. [ Links ]
8. Mubarak M, Kazi JI. Evolution of the diagnostic criteria of antibody-mediated rejection of renal allografts: Banff classification updates. Port J Nephrol Hypert 2013;27:137-42. [ Links ]
9. Mubarak M, Kazi JI. Evolution of the diagnostic criteria of antibody-mediated rejection of renal allografts: Banff classification updates. Port J Nephrol Hypert 2013;27(3):137-142. [ Links ]
10. Weening JJ. The art of classifying renal allograft pathology. Nat Clin Pract Nephrol 2008;4(8):420-421. [ Links ]
11. Solez K. History of the Banff classification of allograft pathology as it approaches its 20th year. Curr Opin Organ Transplant 2010;15(1):49-51. [ Links ]
12. Solez K, Racusen LC. The Banff classification revisited. Kidney Int 2013;83(2):201-206. [ Links ]
13. Solez K, Axelsen RA, Benediktsson H, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 1993;44(2):411-422. [ Links ]
14. Solez K, Benediktsson H, Cavallo T, et al. Report of the third Banff Conference on allograft pathology (July 20-24, 1995) on classification and lesion scoring in renal allograft pathology. Transplant Proc 1996;28(1):441-444. [ Links ]
15. Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999;55(2):713-723. [ Links ]
16. Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 2003;3(6):708-714. [ Links ]
17. Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant 2004;4(10):1562-1566. [ Links ]
18. Solez K, Colvin RB, Racusen LC, et al. Banff 05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (CAN). Am J Transplant 2007;7(3):518-526. [ Links ]
19. Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008;8(4):753-760. [ Links ]
20. Sis B, Mengel M, Haas M, et al. Banff 09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 2010;10(3):464-471. [ Links ]
21. Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting report: new concepts in antibodymediated rejection. Am J Transplant 2012;12(3):563-570. [ Links ]
22. Farris AB, Adams CD, Brousaides N, et al. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol 2011;22(1):176-186. [ Links ]
Prof. Dr. Muhammed Mubarak
Histopathology Department,
Sindh Institute of Urology and Transplantation
Karachi-74200, Pakistan,
E-mail: drmubaraksiut@yahoo.com
Conflict of interest statement: None declared
Received for publication: 11/11/2013
Accepted:12/01/2014














