Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.3 Lisboa Sept. 2014
CASE REPORT
Rhupus syndrome: a case report and literature review
Síndroma de Rhupus: um caso clínico e revisão da literatura
Hernani Goncalves1, Sara Querido1, Flora Rico Sofia1, Carla Gil2, Antonio Patricio1, Sequeira Andrade1
1 Serviço de Nefrologia do Centro Hospitalar do Médio Tejo E.P.E, Unidade de Torres Novas. Torres Novas, Portugal.
2 Serviço de Medicina Interna do Centro Hospitalar do Médio Tejo E.P.E.,Unidade de Torres Novas. Torres Novas, Portugal.
ABSTRACT
The authors describe the case of a 30-year-old female patient with polyarticular juvenile rheumatoid arthritis, who presented with an upper respiratory infection, vomiting and diarrhoea, followed by lower limb and facial oedema and oliguric renal failure. She was admitted to the Nephrology ward of the Centro Hospitalar do Médio Tejo for clinical stabilization and aetiological study. Complementary tests, including immunological study and renal biopsy, led to the diagnosis of Lupus nephritis with rapidly progressive glomerulonephritis.
The authors present a brief revision of systemic lupus erythematosus and of the pathological entity that associates characteristics of lupus and rheumatoid arthritis, known as rhupus, that still remains controversial nowadays.
Key words: Haemodialysis; immunosuppression; lupus nephritis; rheumatoid arthritis; rhupus; systemic lupus erythematosus.
RESUMO
Os autores descrevem o caso clínico de uma doente de 30 anos, com artrite idiopática juvenil poliarticular, que apresentou um quadro de infecção respiratória das vias aéreas superiores, vómitos, diarreia e posteriormente oligoanúria com consequente edema dos membros inferiores e da face. Internada no Serviço de Nefrologia do Centro Hospitalar do Médio Tejo para estabilização clínica e estudo etiológico da doença renal oligoanúrica. Efectuou vários exames complementares incluindo estudo imunológico e biopsia renal, tendo chegado ao diagnóstico de nefrite lúpica com glomerulonefrite crescêntica. Faz-se uma breve revisão sobre lúpus eritematoso sistémico e sobre esta entidade patológica que associa características de lúpus e artrite reumatóide, denominada rhupus, tão envolta em controvérsia.
Palavras chave: Artrite reumatóide; hemodiálise; imunossupressão; lúpus eritematoso sistémico; nefrite lúpica; rhúpus.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by multiple symptoms and clinical findings. Arthralgia and arthritis are common and premature findings. Their most common characteristics are non-erosive deforming lesions, that initially strikes the hands articulations, possibly reaching the synovium. Other findings include vasculitis, serositis, haematological, skin and kidney disorders1-3.
For the diagnosis of rhupus syndrome, erosive polyarthritis and clinical symptoms of SLE, as well as positive dsDNA or anti-Sm autoantibodies are necessary4,5.
In the 1960s and 70s, it was verified that some patients with an initial diagnosis of rheumatoid arthritis (RA) showed erosive arthritis and developed symptoms or signs of SLE, and vice-versa6. Schur (1971) applied for the first time the name rhupus to describe the disease in patients who presented concomitantly characteristics of both diseases7.
Since those decades, a big controversy exists regarding the definition of rhupus as a diagnosis.
Some authors defend that rhupus is an independent entity, while others defend that it is a subtype of SLE, or even an overlapping syndrome of SLE and RA, due to the presence of RA specific antibodies in these patients8,9.
CASE STUDY
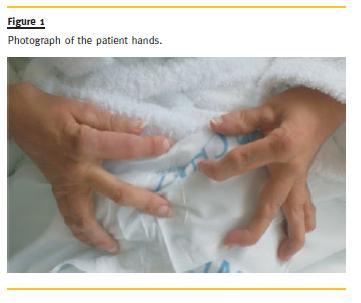
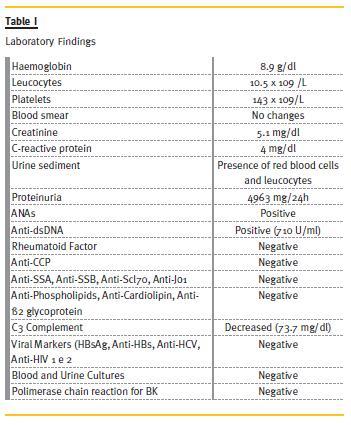
The patient is a 30-year-old female patient, with polyarticular juvenile idiopathic arthritis since the age of 4, pulmonary tuberculosis in 2002,nephrolithiasis and bilateral total hip arthroplasty, with normal renal function, and usually medicated with naproxen, diclofenac and omeprazole. She was admitted, in May 2010, in the Medicine Department of Tomars Hospital, with an infection in the upper airway, vomiting and diarrhoea, which started 2 weeks before admission. Afterwards, symptoms of oliguria and oedema of the lower limbs and face appeared. In the physical exam, she presented lower limbs oedema up to the root of the thighs, face oedema, deformed articulations of the hands and feet (Fig.1), with functional limitation. The neurologic exam was normal. The patient was thin (41 kg), feverish (38ºC), with normal arterial pressure, and an auscultation compatible with stasis. She was transferred to the Nephrology Department of Torres Novas Hospital for compensation and aetiological investigation of the oliguric renal disease. From the study carried out, it was noted severe renal dysfunction (creatinine: 5.1mg/dl, urea: 139mg/dl), normocytic anaemia (haemoglobin: 8.9 g/dl), leukocytosis (10.5 x 109/L) thrombocytopenia (143x109/L), no changes in blood smears, elevated C-reactive protein (CRP) (4 mg/dl), nephrotic-range proteinuria (4963mg/24h in 400 ml of diuresis) and urine sediment with red blood cells and leukocytes. Screening for autoimmune diseases revealed positive ANAs and strongly positive anti-dsDNA (710 U/ml). Rheumatoid factor, anti-CCP antibodies, anti-SSA, anti-SSB, anti-Scl70, anti-Jo1, anti-GBM, anti-phospholipids, anti-cardiolipin and anti-β 2 glycoprotein were negative. Complement levels were decreased (C3: 73.7mg/dl). Viral markers (HBsAg, anti-HBs, anti-HCV, anti-HIV 1 and 2) were negative. During the hospitalization, blood and urine cultures were negative and polymerase chain reaction for BK in sputum was also negative (Table I).
Renal ultrasound showed normal kidneys dimensions, with maintained differentiation and thickness of the parenchyma, without pielo-caliceal dilatation.
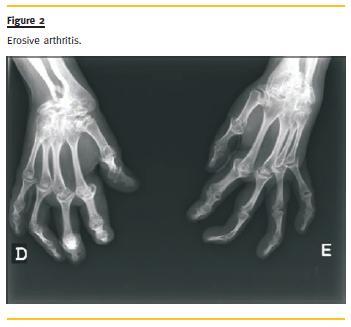
The echocardiogram showed good global systolic function, without pericardial effusion, and the thoracic radiography revealed bilateral pleural effusion. The pulmonary CT confirmed intra-cisural and bilateral pleural effusion, hilar vascular engorgement with thickening of the pulmonary interstitium in the upper lobes with small parenchymal infiltrates. The radiography of the hands showed erosive arthritis (Fig.2).
The patient started large spectrum antibiotics for the upper respiratory tract infection, but the fever persisted. Urgent haemodialysis was also performed due to severe impairment of the renal function, with oliguria and fluid overload.
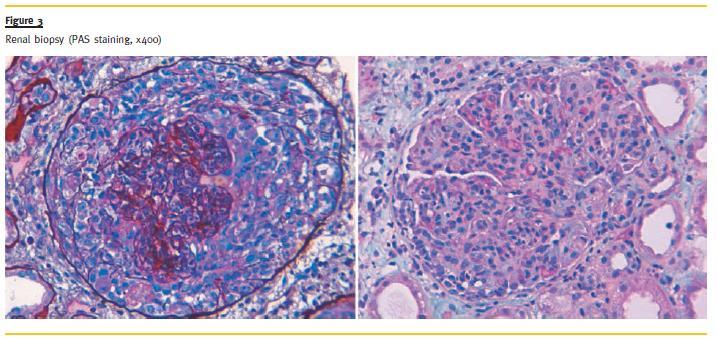
A kidney biopsy was performed on the 4th day of admission and it revealed the presence of endocapillar proliferative glomerulonephritis, with crescent formation in all glomeruli, Bowmans capsule rupture and acute tubular necrosis consistent with a diagnosis of lupus nephritis class IV (Fig.3). It was not possible to perform immunofluorescence technique due to the lack of kidney tissue.
She started immunosuppressive treatment with 3 pulses of methylprednisolone 500 mg IV and 3 administrations of cyclophosphamide 0.5 g/m2/month, with an interval of three months. After that, the patient remained afebrile.
She was discharged at the end of 3 weeks of hospitalization, medicated with prednisolone 1mg/kg, isoniazid, pyridoxine, calcium carbonate, esomeprazole and darbepoetin, She kept on a regular haemodialysis programme, three times a week. Three months after the hospitalization, partial recuperation of the renal function was verified, leading to haemodialysis discontinuation. In November 2010, mycophenolate mofetil 500 mg twice a day was started.
At present, the patient is asymptomatic, with creatinine clearance greater than 90ml/min, negative anti-dsDNAand ANA, normal C3 complement, while maintaining proteinuria less than 1g/24h and no haematuria.
DISCUSSION
We present a case of RA with symmetrical and erosive joint involvement of the extremities, in a 30-year-old female patient, followed, after a long period, by clinical and laboratory features of lupus.
The last diagnosis was based on pleural effusion, renal and haematological involvement, positive ANA and anti-dsDNA. According to the literature, the combination of these diseases corresponds to rhupus4,5.
This case is singular, because in the cases of rhupus, usually, we find cutaneous and haematological abnormalities, and not such severe renal involvement.
Systemic lupus erythematosus is a systemic autoimmune disease, in which the tissues and the cells are damaged by antibodies and immunocomplexes.
The female gender is the most affected (90%), with a peak incidence between the ages of 25-351-3.
The aetiology and pathogenesis of SLE remain uncertain, however, it is known that the disease is caused by the interaction of the genes that are found in the HLA region, particularly HLA class II DR and DQ and class III encoding the complement C2 and C4, and environment factors, that result in an abnormal immunological response, with incessant production of antibodies and formation of immunocomplexes1,3.
These fixate in the target tissues, destroying them, releasing chemotaxins, proteolytic enzymes and vasoactive peptides, causing a chronic inflammation. Many antibodies take part in this phenomenon, among them: DNA antibodies (ANA, antidsDNA antibodies), anti-RNA (anti-Sm, anti-RNP, anti-SSB, anti-SSA), anti-histone antibodies, antiphospholipid, anti-erythrocyte, anti-platelet and anti-ribossomic1-3.
The diagnosis of SLE is based on the clinical manifestations and the presence of autoantibodies, where the presence of at least 4 out of 10 criteria, according the American College of Rheumatology (Table II), is necessary3. In this case, the patient had haematological and renal abnormalities, and positive ANA and anti-dsDNA antibodies, 4 criteria for the diagnosis of SLE.
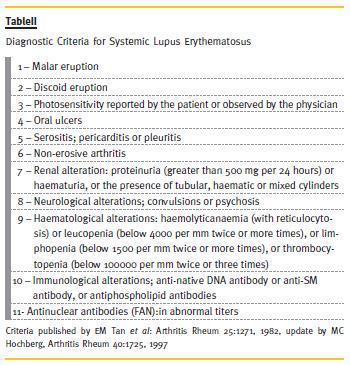
The SLE manifestations are different, depending on the organ or tissue affected. These can include skin, kidney, joints, vessels, eyes and neurological, pulmonary, cardiac, haematological and gastrointestinal systems1-3. Renal manifestations occur in 40-85% of patients, due to immunocomplex deposition in the glomeruli. These may present as changes in urinary sediment (proteinuria, microscopic haematuria), nephritic syndrome, nephrotic syndrome, failure3.
Lupus nephritis is an immune complex glomerulonephritis with decreased levels of C3, with positive ANA and anti-dsDNAantibodies most of the times10.
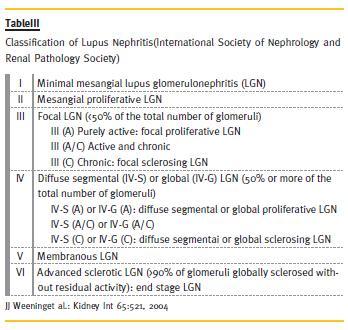
The presence of the three immunoglobulins,IgG, IgA and IgM and complements C1q and C3, in the fluorescence microscopy known as full house is highly suggestive of lupus nephritis4,10. However, significant anatomical-clinical dissociation is usually found, with clinical and laboratory elements not being able to predict the severity of renal histology. Thus, it is usually necessary to perform a renal biopsy and this remains the gold-standard for the diagnosis and selection of the treatment10. According to the International Society of Nephrology, lupus nephritis can be classified into six classes according to the histology 3,10 (Table III).
Commonly, at age 20s the survival rate of SLE rounds 75%, however, the occurrence of severe lupus nephritis is associated with poor prognosis, achieving a mortality of about 50% in 10 years10.
Joint involvement is typically the first manifestation that occurs in SLE, with multiple presentations that include arthralgia, non-deforming arthritis, and deforming arthritis with or without erosions. The non-deforming arthritis consists on intermittent polyarthritis, slight to disabling, characterized by soft tissue swelling and joint pain5. The term Jaccoud arthropathy was defined by Brywaters (1950), when he noticed that the deforming arthritis in patients with SLE was very similar to the arthritis that Jaccoud described in 1869, associated to the rheumatic fever5.
This disease is characterized by non-erosive deformations, subluxation of the metacarpophalangeal joints with ulnar deviation, and hyperextension of the proximal interphalangeal joints, which are all reversible at an early stage5.
By contrast, the arthritis found in RA is symmetrical, erosive, and rarely affect the distal interphalangeal joints. Some of the changes that can be found in the joints are: ulnar deviation of the fingers through the wrist abduction and injury of the metacarpal phalangeal (MCP); fingers in swan lap due to the flexion of MCP, hyperextension of the proximal interphalangeal (PIP) and flexion of the distal interphalangeal (DIP), buttonhole fingers due to the flexion of PIP and hyperextension of DIP; Z thumbby flexion of MCP and hyperextension of the interphalangeal; finger pending due to the ruptureof the extensor tendon11.
Despite the highly detailed characterization of these two syndromes as to separate entities, sometimes there are cases in which the characteristics of SLE and RA are found together6,7.
In 1969, Kantor published a case of erosive arthropathy in a patient with SLE and RA6.In the 1970s, Schur used the designation rhupus for the first time to define a new entity that had features of SLE and RA7.From this date, this definition has been discussed many times. Is Rhupus an independent entity, an overlapping syndrome, or a subtype of SLE8,9?
The truth is that the prevalence of erosive arthritis in patients with SLE is low, about 1-5%9. Fernandez, et al., when reviewing and observing 8 cases with rhupus, stated that this entity is a subtype of SLE. They associated, with high probability, the antibody anti-RA33 with the presence of erosive arthritis in SLE8.
On the other hand, Amezcua-Guerra,et al. found high titrations of anti-CCP, defending that this entity consists in an overlapping syndrome9. Amezcua-Guerra, in 2009, affirmed that there is no doubt that rupus belongs to the spectrum of SLE, however, he questioned if these patients also have RA. There is evidence that sustains the existence of rhupus as an overlapping syndrome. The author used three arguments to explain his point of view. The first one is the presence of anti CCP, which is very specific to RA; the second is the established association between the risk of RA and the MHC II molecules, which share the same sequence of amino acids in the 72nd – 74thposition of the β chain. These shared epitope (SE) alleles connect preferentially to the peptides that have as a component the citrulline amino acid, and have an effect on the production of anti-CCP in RA. It is possible that the association between SLE and erosive arthritis is due to the indirect effect mediated by antibodies against different citrullinated proteins. Sixty-seven per cent of patients with SLE and erosive arthritis are carriers of SE alleles, in contrast with the 22% of patients with non-erosive arthritis, and the presence of two copies of SE alleles increase by 8 the risk of erosive arthritis in SLE. The third argument involves CRP. This directly correlates with the activity in RA, with a deficient production in SLE. However, in rhupus a normal production of CRP was verified. The author concluded that the criteria of SLE and RA should be re-evaluated in the light of new discoveries, and remark that autoimmune diseases are classified and artificially differentiated by barriers created by doctors and investigators12.
CONCLUSION
Rhupus is the most probable diagnosis to the presented case. It is very interesting to see that the disease extends for a very long period of time (26 years), and the signs and symptoms are different in different periods of time.
This case is rare, because in the cases of rhupus, usually, we find cutaneous and haematological abnormalities, and not such severe renal involvement. In this patient with severe renal impairment, with an adequate immunosuppressive strategy, renal function recovery was possible which led to an improvement of the quality of life of a young female, already limited by the articular component of the disease.
Although the diagnosis of SLE and RA is relatively frequent, rhupus is a rare entity. Its place in autoimmune pathologies as a distinct entity or an overlapping syndrome is still uncertain.
References
1. Bartels CM, Muller D. Systemic Lupus Erythematosus, Emedic ine, updated 2011 March 8. Available from: www.emedicine.medscape.com [ Links ]
2. Rahman A,Isenberg DA. Systemic lupus erythematosus.NEngl J Med 2008;358(9):929–939 [ Links ]
3. Hahn BH. Systemic lupus erythematosus. In:Fauci AS, Braunwald E, Kasper DL, et al. Harrisons Principles of Internal Medicine. 17thed. New York: McGraw-Hill Medical 2008: 2075-2083 [ Links ]
4. Santos R, Silva R, Malvar B, Pessegueiro P, Pires C.Nephrotic proteinuric in a patient with Rhupus. Port JNephrolHypert 2013;27(4):295-299 [ Links ]
5. Van Vugt RM, Derksen RH, Kater L, Bijlsma JW. Deforming arthropathy or lupus and rhupus hands in systemic lupus erythematosus. AmRheumDis1998;57(9):540-544 [ Links ]
6. Kantor GL, Bickel YB, Barnett EV. Coexistence of systemic lupus erythematosus and rheumatoid arthritis. Report of a case and review of the literature, with clinical, pathologic and serologic observations. Am J Med1969;47(3):433-444 [ Links ]
7. Schur PH. Systemic lupus erythematosus, In: Beeson PB, McDermott W (eds): Cecil- Loeb Textbook of Medicine. Ed 13. Philadelphia:WB Saunders, Co. 1971; 821 [ Links ]
8. Fernandez A, Quintana G, RondónF, et al.Lupus arthropathy: a case series of patients with rhupus. Clin Rheumatoljavascript:AL_get(this, jour, Clin Rheumatol.); 2006;25(2):164-167
9. Amezcua-Guerra LM, Springall R, Marquez-Velasco R, Gómez-Garcia L, Vargas A, Bojalil R. Presence of antibodies against cyclic citrullinated peptides in patients with ruphus: a cross-sectional study. Arthritis Res Ther 2006;8(5):R144 [ Links ]
10. Appel GB, Jayne D. Lupus nephritis. In: Floege J, Johnson RJ, Feehaly J. Comprehensive Clinical Nephrology. 4thed. St. Louis: Elsevier Saunders 2010: 308-321 [ Links ]
11. Smith HR.Rheumathoid arthritis, Emedicine, updated 2010 September 22. Available from: www.emedicine.medscape.com [ Links ]
12. Amezcua-Guerra LM. Overlap between systemic lupus erythematosus and rheumatoid arthritis: Is it real or just an illusion? J Rheumatol 2009;36(1):4-6 [ Links ]
Dr. Hernâni Ricardo Martins Gonçalves
Serviço de Nefrologia do Centro Hospitalar do Médio Tejo E.P.E, Unidade de Torres Novas.
Avenida Xanana Gusmão, Apartado 45, 2350-754 Torres Novas, Portugal
E-mail: hrmg22@gmail.com
Conflict of interest statement: None declared.
Received for publication: 27/11/2013
Accepted in revised form: 04/08/2014














