Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.1 Lisboa Mar. 2015
ORIGINAL ARTICLE
Monitoring the conversion from brand name to generic mycophenolate mofetil in kidney transplant recipients
Monitorização da conversão de micofenolato de mofetil original a genérico em transplantados renais
Rita Gouveia1, Cristina Outerelo1, Isabel Carvalho2, Pedro Bravo1, Pedro Cruz1, Ana Mateus1, Carlos Oliveira1, Aura Ramos1
1 Department of Nephrology, Kidney Transplant Unit and
2 Department of Clinical Pathology, Hospital Garcia de Orta. Almada, Portugal
ABSTRACT
Generic mycophenolate mofetil (MMF) is a cheaper and possibly equally effective option as the original molecule for maintenance immunosuppression in solid organ transplant recipients. In order to be approved, each generic formulation has to prove bioequivalence to the reference product in healthy volunteers. However, MMF pharmacokinetics may be different between healthy volunteers and transplant recipients. Faced with the introduction of the generic formulation in the pharmacy of their kidney transplant unit, the authors studied and compared the exposure to mycophenolic acid (MPA) before and after the switch in 14 kidney transplant recipients converted to the generic molecule. The exposure to MPA as evaluated by the AUC0-12h estimated by a limited sample strategy was slightly higher with the generic formulation (40.49 versus 34.96mcg.h/mL, p = 0.041). There was no significant variation in the eGFR, haemoglobin and leucocyte count after the switch. Only one of the patients converted to the generic formulation developed de novo adverse gastrointestinal effects, which resolved after restart of the original formulation. In conclusion, in our cohort there was no clinically significant variation in the exposure to MPA, kidney graft function or incidence of adverse events with the generic formulation on a short follow-up period.
Key-words: Conversion; generic; immunosuppression; mycophenolate; kidney transplant; tacrolimus.
RESUMO
O micofenolato de mofetil (MMF) genérico é uma alternativa mais barata e possivelmente tão eficaz quanto a molécula original na imunossupressão de manutenção em receptores de transplantes de órgãos sólidos, nomeadamente transplante renal. Para ser aprovada, cada nova formulação genérica tem que demonstrar bioequivalência ao fármaco original em voluntários saudáveis. No entanto, a farmacocinética do MMF não é necessariamente igual em voluntários saudáveis e receptores de transplantes. Perante a imposição da conversão dos doentes para MMF genérico os autores avaliaram e compararam exposição ao ácido micofenólico (AMF) com o fármaco original e com o genérico em 14 doentes convertidos ao genérico na sua unidade de transplante renal. A exposição ao AMF, avaliada pela AUC0-12h estimada a partir de 3 determinações séricas em tempos pré-definidos, foi ligeiramente mais elevada com a formulação genérica (40.49 versus 34.96mcg.h/mL, p = 0,041). Não se verificou variação significativa da TFGe, hemoglobina nem contagem de leucócitos após a conversão para o fármaco genérico. Apenas um dos doentes convertidos para MMF genérico desenvolveu efeitos adversos do foro gastrointestinal de novo que resolveram após reintrodução da molécula original. Em conclusão, na nossa amostra não se verificou variação clinicamente significativa na exposição ao AMF, função do enxerto renal nem na incidência de efeitos adversos com a formulação genérica, a curto prazo.
Palavras-chave: Conversão; genérico; imunossupressão; micofenolato; tacrolimus; transplante renal.
INTRODUCTION
Kidney transplant is the kidney replacement therapy of choice in eligible patients given its survival and quality of life benefits compared with other options. However, among other factors, the associated costs related with the need for lifelong immunosuppression may prevent patients access to transplant.
Therefore, as the patents of the older immunosuppressive medications expire, there has been increasing interest in the use of generic ones given the cost saving potential, namely in kidney transplant. Mycophenolate is recommended as a first-line antiproliferative agent for maintenance therapy in kidney transplant1. Mycophenolic acid is the active metabolite responsible for the immunosuppressive effects.
The registration of generic drugs is based on the demonstration of bioequivalence to the reference one in healthy volunteers. This means that in pharmacokinetic studies the ratio of the geometric means of the generic to reference drug pharmacokinetic parameters of peak concentration and area under the concentration–time curve in one dosing interval (AUC0-T) and AUC0-72h must have a 90% confidence interval within the 80-125% range2. However, bioequivalence in healthy volunteers does not guarantee therapeutic equivalence in the kidney transplant population.
A bioequivalence study in kidney transplant recipients has shown that the steady state pharmacokinetics of mycophenolic acid (MPA) after administration of a generic form of mycophenolate mofetil (MMF) or the originator drug is comparable in stable kidney allograft recipients3. In this study, the adverse events profile was similar between the two treatment groups. Another study has also shown similar MPA exposure with generic MMF, with no need for dose adjustments and stable graft function4.
The need for concentration controlled dosing and how MPA exposure is best measured in clinical practice is also a subject of debate5. The majority of studies analysing the relationship between MPA exposure and clinical outcomes have shown a good correlation between the full total MPA AUC0-12h and the risk of rejection, at least early after transplantation6-7. However, the determination of the full AUC profile requires multiple frequ en t sampling for determinations of the MPA concentration during a complete dosing interval (usually 12 hours), making it impractical in the clinical setting5. Unlike other drugs, MPA concentration at single time points either immediately before or at exact times after dosing have a weak association not only with the full AUC5, but also with clinical outcomes6.
Several investigators have shown that a good estimate of MPA AUC0-12h can be obtained by a limited sampling strategy in the first 2 to 4 hours after administration of the drug. Algorithms with acceptable predictive performance have been proposed for kidney transplant recipients and for regimens that include MMF plus cyclosporine, tacrolimus or sirolimus8.
Under cost saving constraints, all patients in our kidney transplant unit were switched to a generic MMF formulation. In the present study we aimed to compare the exposure to mycophenolic acid between the original and the generic molecule in a cohort of our patients, to evaluate the efficacy and safety of the conversion to generic MMF.
METHODS
Between May and July 2012, all kidney transplant recipients under maintenance immunosuppression with the original MMF in our unit were converted to the generic molecule, on a 1:1 dose. Generic MMF was always supplied by Generis®.
We studied the first 14 patients to be converted who were, at least, 12 months post-transplantation, with stable kidney graft function (no increase in serum creatinine from baseline of more than 0.3mg/dL in the previous 3 months) and willing to participate in the study. Each of these 14 patients underwent blood sampling in the unit at two time points: the first while taking the original molecule and the second 3 weeks after conversion to the generic MMF.
The protocol for determination of the MPA AUC0-12h was the following: blood samples for MPA analysis were collected immediately before (C0h) administration of the drug and at 30 minutes (C0.5h) and 2 hours (C2h) after administration.
These samples were immediately centrifuged, frozen and stored at -20 °C until analysis. MPA plasma concentrations were measured using an enzymatic method. The AUC0-12h was calculated using the following formula9: AUC0-12h = 7.75 + 6.49C0h + 0.76C0.5h + 2.43C2h.
We collected patient data concerning demographics, graft function as estimated by de 4 variable Modification of Diet in Renal Disease equation (eGFR), through levels of calcineurin or mTOR inhibitors, haemoglobin and leucocyte count immediately before and 3 weeks after conversion. Any adverse events during the period of the study were registered.
Additionally, an estimate on the costs related to MMF prescription was obtained before and after the conversion. The statistical analysis comparing the values before and after the conversion was done using a paired T-test.
RESULTS
All 14 patients completed the protocol for determination of MPA AUC0-12h before and after the conversion.
A total of 28 determinations of AUC0-12h were obtained with the original and the generic molecule. There was no change in the MMF dose for each patient during the study (500mg to 2g/day). The baseline characteristics of the patients are presented in Table I. There was no variation of the doses or levels of the calcineurin and mTOR inhibitor during the study. There was also no change in the additional medication. The results of the mycophenolic acid determinations are shown in Table II. There was no significant variation in the eGFR, haemoglobin or leucocyte count after the switch (Table III). Only one of the patients converted to generic MMF developed de novo adverse gastrointestinal effects, which resolved after restart of the original formulation. In this patient, although there was no significant variation of the AUC0-12h (69 mcg.h/mL with both formulations), C0.5h was significantly higher with the generic formulation (24 versus 11.8mcg/mL).
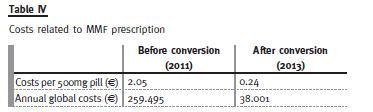
The costs related to MMF prescription in our unit are presented in Table IV, showing a reduction both in cost per pill and in global costs.
DISCUSSION
We have undertaken a small prospective study comparing pharmacokinetic variables and graft function before and after conversion to a generic formulation of MMF in selected stable kidney transplant recipients at a single centre.
An association between MPA exposure, especially when evaluated by either limited sampling or full AUC0-12h, and the risk of acute rejection has been shown in observational and randomized trials5,7-9.
According to their results, a therapeutic window of 30 to 60 mcg.h/mL has been proposed for patients with low to intermediate immunologic risk and who are on calcineurin inhibitors based therapy5. It is worth mentioning that these trials enrolled patients in the first 3 months post-transplantation. Data including patients in a later post-transplantation period or under calcineurin inhibitors minimization/withdrawal protocols are lacking.
Our data showed a slightly higher exposure to mycophenolic acid with the generic formulation compared to the original one, which was statistically significant. Nevertheless, most patients showed an MPA exposure within the target range with both MMF formulations, suggesting that the difference observed has no clinical implications. This is supported by the absence of significant variation of the graft function measured by eGFR (MDRD) or haematologic parameters (haemoglobin and leucocyte count) during the study period.
Only one of the patients that were switched to the generic formulation developed de novo gastrointestinal complaints. After restart of the original formulation the symptoms subsided. Contradictory results have been reported on the relationship between MPA exposure and adverse events.
Although larger studies have shown no correlation5,7-9, between MPA predose trough concentration or AUC0-12h and MMF related adverse events, one study has reported an association between higher MPA concentrations at 30 minutes after MMF dosing and adverse events10. The only patient in our population that developed an adverse reaction attributable to generic MMF, namely gastrointestinal intolerance, showed a higher MPA concentration at 30 minutes with the generic formulation, with the same AUC0-12h.
From an economical point of view, there were considerable cost savings with the introduction of the generic formulation. The conversion allowed an 88% reduction on the cost per 500mg pill and an 85% reduction on the global annual costs with MMF prescription in our centre.
This study has several limitations, namely the small number of patients enrolled, the short followup period and the fact that this protocol for determination of the exposure to MPA is impractical in our daily practice. The question remains on whether this pharmacokinetic overlap in kidney transplant recipients translates into therapeutic equivalence.
Larger studies with longer follow-up are needed to assess the long-term effectiveness and safety of the generic drug.
CONCLUSION
In our cohort, there was a statistically significant increase in the exposure to MPA with the generic formulation of MMF. Nevertheless, most patients showed an MPA exposure within the target range with both MMF formulations and stable graft function and haematologic parameters after the switch. Only one patient had to suspend generic MMF because of de novo adverse gastrointestinal symptoms. The conversion to generic MMF resulted in significant cost-savings. Despite the small number of patients included in the study, our data suggest that the switch to generic MMF may be safe and effective in stable kidney transplant recipients, at least on the short term.
Additional studies to evaluate the long-term safety and cost-effectiveness of generic MMF conversion are warranted.
References
1. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9 Suppl 3:S1-155. [ Links ]
2. Guideline on the investigation of bioequivalence. CPMP/EWP/QWP/1401/98 Rev. 1/ Corr (London, 20 January 2010) [ Links ]
3. Sunder-Plassmann G, Reinke P, Rath T, et al. Comparative pharmacokinetic study of two mycophenolate mofetil formulations in stable kidney transplant recipients. Transpl Int 2012;25(6):680-686. [ Links ]
4. Videla C, Godoy C. Converting to a generic formulation of mycophenolate mofetil in stable kidney transplant recipients: 1 year of drug surveillance and outcome. Transplant Proc 2007;39(3):602-605. [ Links ]
5. Kuypers DR, Le Meur Y, Cantarovich M, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol 2010;5(2):341-358. [ Links ]
6. Knight SR, Morris PJ. Does the evidence support the use of mycophenolate mofetil therapeutic drug monitoring in clinical practice? A systematic review. Transplantation 2008;85(12):1675-1685. [ Links ]
7. van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 1999;68(2):261-266. [ Links ]
8. Le Meur Y, Büchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant 2007;7(11):2496-24503. [ Links ]
7. van Gelder T, Silva HT, de Fijter JW, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation 2008;86(8):1043-1051. [ Links ]
10. Shaw LM, Figurski M, Milone MC, Trofe J, Bloom RD. Therapeutic drug monitoring of mycophenolic acid. Clin J Am Soc Nephrol 2007;2(5):1062-1072. [ Links ]
11. Pawinski T, Hale M, Korecka M, Fitzsimmons WE, Shaw LM. Limited sampling strategy for the estimation of mycophenolic acid area under the curve in adult renal transplant patients treated with concomitant tacrolimus. Clin Chem 2002;48(9):1497-1504. [ Links ]
12. Mourad M, Malaise J, Eddour DC, et al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem 2001;47(1):88-94. [ Links ]
Drª Rita Gouveia
Department of Nephrology, Kidney Transplant Unit
Hospital Garcia de Orta
Avenida Torrado da Silva, 2801-951 Almada, Portugal
E-mail: ritagouveia18@msn.com
Conflict of interest statement: None declared.
Received for publication: 10/10/2014
Accepted in revised form: 9/01/2015














