Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Portuguese Journal of Nephrology & Hypertension
versión impresa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.2 Lisboa jun. 2015
EDITORIAL
Standard and novel therapeutic approaches to diabetic nephropathy
Jesus Egido, Beatriz Fernandez-Fernandez, Alberto Ortiz, Sebastian Mas, Carmen Gómez-Guerrero
Division of Nephrology and Hypertension. Renal, Vascular and Diabetes Research Laboratory. Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders (CIBERDEM) and Renal Foundation FRIAT
University Hospital Fundacion Jimenez Diaz, Autonoma University, Madrid, Spain.
INTRODUCTION
Diabetes is a significant international health care problem. The prevalence of diabetes mellitus has reached epidemic proportions. Worldwide, around 347 million people have the disease and this number is expected to increase to 430 million by 2030. The disease comes with a financial burden. An estimated US$548 billion was spent on this condition in 2013.
The vast majority of this cost relates to the management of diabetic complications. Vascular injury and comorbidities influence the natural history of the disease, particularly in patients with type 2 diabetes mellitus (T2DM). The 1988-1994 NHANES III survey (n = 14,622) reported that kidney disease (defined as albuminuria, decreased renal function or both) was present in 42% of US patients with diabetes1.
Patients with diabetic kidney disease are at increased risk of cardiovascular events and mortality.
Diabetes is the most fre-quent cause of end-stage renal disease (ESRD) in developed countries, accounting for 25–40% of incident patients. The classic pattern of diabetic nephropathy involves initial microalbuminuria [urinary albumin excretion rate (UAER) 30–300 mg/24 h] that progresses to macroalbuminuria (UAER ≥ 300 mg/24 h) and is followed by a progressive decrease in renal function leading to ESRD. Around 25-35% of patients with diabetes and decreased renal function have normoalbuminuria or microalbuminuria, raising new questions about the mechanisms that contribute to kidney damage and potential therapeutic targets2.Several hypotheses have been proposed to attempt to explain progression of diabetic kidney disease in patients with normoalbuminuria or microalbuminuria. Incomplete recovery from repetitive episodes of acute kidney injury (AKI), haemodynamic changes and renal vascular disease might be contributing factors. Both hyperuricaemia and systemic inflammation are associated with progression of non-proteinuric diabetic kidney disease3-6.
Unless novel, effective therapeutic approaches to diabetic kidney disease are developed, the expected worldwide increase in associated cases of ESRD and cardiovascular disease will likely overwhelm healthcare resources, leading to a human tragedy of major proportions.
REFINEMENT OF STANDARD THERAPIES
Blood pressure control and ACEi/ARB treatment
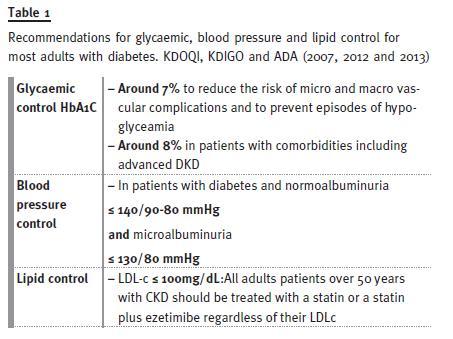
The 2007 KDOQI guidelines for diabetes and chronic kidney disease (CKD) recommended a blood pressure target for patients with diabetic kidney disease of ≤130/80 mmHg6. However, the 2012 KDIGO guidelines recommend a target of ≤140/90 mmHg for normoalbuminuric patients with diabetes because the ACCORD study7 disclosed evidence of harm with tight blood pressure control in elderly patients (mean age 62 years) and in patients with comorbidities, marked systolic hypertension or severe autonomic neuropathy. The current blood pressure target recommended by KDIGO for the management of blood pressure in diabetic patients with CKD and albuminuria is ≤130/80 mmHg (8;9) By contrast, 2014 ADA guidelines recommend that blood pressure should be <140/80 mmHg, although in young patients or pregnant women a systolic blood pressure target of <130 mmHg could be suitable. Patients with long life expectancies are considered to be candidates for lower blood pressure goals than older patients because of the beneficial effects of lowering blood pressure on albuminuria.
Use of angiotensin converting enzyme (ACE) inhibitors or angiotensin II type 1 receptor blockers (ARBs) is the current standard strategy to control blood pres-sure, reduce proteinuria and slow the loss of renal function in patients with diabetic kidney disease.
The revised 2014 American Diabetes Association (ADA) guidelines for standards of medical care in diabetes recommend ACE inhibitors or ARBs for the treatment of patients with microalbuminuria or macroalbuminuria9.
In normoalbuminuric patients with T2DM and hypertension, renin-angiotensin system (RAS) blockade delays the development of microalbuminuria.
However, neither ARBs nor ACE inhibitors have been shown to prevent microalbuminuria in normotensive patients with T1DM. The 2012 KDOQI guidelines for diabetes and CKD10 and the 2014 ADA guidelines9,therefore, do not recommend using ACE inhibitors or ARBs for the primary prevention of diabetic kidney disease in normotensive patients with normoalbuminuria.
Dual blockade of the RAS (ACE inhibitor plus ARB) might decrease proteinuria more effectively than single RAS blockade (ACE inhibitor or ARB). However, this strategy is not recommended by the 2012 KDOQI, 2012 KDIGO or 2014 ADA guidelines as no additional efficacy in terms of renal function has been demonstrated and the incidence and severity of adverse effects is increased. The ALTITUDE11 and VA NEPHROND12 randomized controlled trials showed no beneficial effects of dual versus single RAS blockade on renal function and both studies were stopped prematurely for safety reasons.
Ongoing randomized controlled trials are now investigating the effects of combining RAS blockade with mineralocorticoid receptor antagonists (MRAs), which target aldosterone actions.
Metabolic control
Metabolic control in patients with diabetic kidney disease requires optimization of glucose and lipid levels. Hyperglycaemia is the driving force for diabetic complications, whereas hyperlipidaemia contributes to increased cardiovascular mortality13. Optimized metabolic control, new antidiabetic agents (such as incretin-based therapies), body weight reduction and statins might have a role in a holistic approach to diabetic kidney disease.
The 2014 ADA guidelines recommend an HbA1c target of ~7% to reduce the risk of microvascular and macrovascular complications and to avoid potentially life-threatening episodes of hypoglycaemia-9 However, the guidelines state that HbA1c levels of ~8% are admissible in patients with comorbid conditions and advanced microvascular or macrovascular disease who have difficulty in achieving tighter glycaemic control. This group might include patients with advanced-stage diabetic kidney disease.
Novel drugs controlling blood glucose
Treatments that target the incretin system might improve glucose control and decrease microvascular and macro-vascular complications in patients with diabetes. The incretins-gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) are released from gut cells in response to food intake. They suppress gluca-gon secretion and stimulate insulin secretion, therefore, reducing serum glucose levels14.
As integrins are degraded by dipeptidyl peptidase-4 (DPP4), agents that target the incretin system include DPP4 inhibitors and DDP4-resistant peptides that activate the GLP-1 receptor14. The DPP4 inhibitors (sitagliptin, vildagliptin, saxagliptin, linagliptin and alogliptin) and GLP-1 agonists (exenatide and liraglutide) can improve glucose and weight control in patients with T2DM and are associated with a low risk of hypoglycaemia.
Data from animal models suggest that DPP4 inhibitors might improve diabetic kidney disease at doses that do not improve glycaemic control, but these observations have not yet been confirmed in humans To shed light on this issue, randomized controlled trials comparing incretin mimetics with other antidiabetic drugs using the primary outcome of GFR or hard renal end points are required.
Inhibitors of sodium-glucose co-transporter type 2 (SGLT2), such as canagliflozin and dapagliflozin, are recently approved for treatment of type 2 diabetes.
These agents lower blood glucose mainly by increasing urinary glucose excretion. Advantages of this drug class include modest weight loss of approximately 2 kg, low-risk of hypoglycaemia, and decreased blood pressure of approximately 4 mmHg systolic and 2 mmHg diastolic. These characteristics make these agents potential add-on therapy in patients with HbA1c levels close to 7%-8.0%, particularly if these patients are obese, hypertensive, and/or prone for hypoglycaemia. Overall, SGLT2 inhibitors are useful addition for treatment of select groups of patients with type 2 diabetes, but their efficacy and safety need to be established in long-term clinical trials15.
Body weight reduction
As obesity precedes and is strongly associated with diabetes, weight reduction is considered a first step in com-bating T2DM and its complications. In obese patients with T2DM, weight loss has been shown to reduce albuminuria in the early stages of diabetic kidney disease(16). Similarly, in obese individuals with advanced diabetic kidney disease (eGFR < 40 ml/min/1.73 m2 and UAE > 30 mg per day), a 12-week, very low calorie, ketogenic weight-reduction diet with encouragement of exercise led to a 12% reduction in weight, a 36% reduction in albuminuria and statistically significant reductions in serum cre-atinine and cystatin C levels, as well as an improvement in glycaemic control.
Bariatric surgery can reduce hypertension, albuminuria and inflammation, and might be a therapeutic option for obese patients with diabetes and macroalbuminuria17.
To date, no studies have assessed the effects of bariatric surgery on albuminuria in these patients.
Lipid management
Patients with T2DM have an increased prevalence of lipid abnormalities, which contribute to their high risk of cardiovascular disease9,18. The dyslipidaemia pattern in diabetes includes hypertriglyceridemia, low HDL levels and increased levels of small, dense LDL particles and oxidized LDL cholesterol18. The 2013 KDIGO guide-lines for lipid management in CKD recommend that all adults with diabetes and CKD should be treated with a statin or a statin plus ezetimibe regardless of their LDL cholesterol levels.
Overall, the evidence for beneficial effects of statins in patients with diabetes and a moderate or high risk of cardiovascular disease are convincing.
Numerous trials have confirmed the cardiovascular benefits of this therapy. Evidence from some small trials suggests that statin treatment might also delay the progression of dia-betic kidney disease.
The present recommendations for glycaemic, blood pressure and lipid controls are depicted in Table 1
NOVEL APPROACHES BEYOND METABOLIC CONTROL
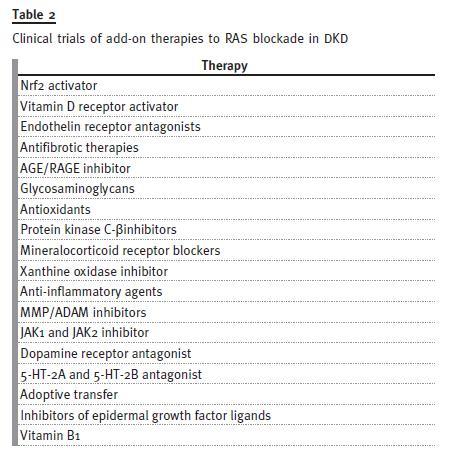
In the past few years, a series of randomized controlled trials that investigated new approaches to diabetic kidney disease have provided negative or inconclusive data or were terminated due to safety concerns or a lack of effi-cacy (Table 2).
Nrf2 activators
Bardoxolone methyl is a synthetic triterpenoid that activates the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription pathway and inhibits nuclear factor κB (NF-κB)19. The BEAM phase II placebo bardoxolone therapy increased eGFR by 5.8–10.5 ml/min/1.73 m2 at 24 weeks, and this improvement persisted at week 52.However, the BEACON phase III randomized controlled trial, which aimed to confirm the efficacy and safety of bardoxolone methyl, was terminated early due to safety concerns20.
Vitamin D receptor (VDR) activators
In humans, vitamin D deficiency is associated with inflammation, immune dysfunction, endothelial dysfunction and cardiovascular disease.
Interestingly, podocytes express VDRs and their activation can directly decrease the inflammatory response of these cells to high glucose level21. In animal models of diabetes, VDR deficiency increased albuminuria, whereas treatment with the VDR activators calcitriol or paricalcitol had antiproteinuric and anti-inflammatory effects21,22 Paricalcitol has some clinical advantages over the natural VDR ligand calcitriol in terms of adverse effects on serum calcium and phosphorus levels. However, the VITAL phase III randomized controlled trial, which investigated the anti-proteinuric effect of paricalcitol (1 μg or 2 μg per 24 h) as an adjuvant therapy to RAS blockade in 281 patients with T2DM and stages 2–4 CKD, showed no significant difference between the intervention and placebo groups in the primary end point of percentage change in UACR at 24 weeks (between-group difference for paricalcitrol versus placebo of –15%, 95% CI –28% to 1%, P = 0.071)23. At 24 weeks, the secondary outcome of 24-hour albuminuria decreased significantly in the 2 μg paricalcitol group (–717 mg versus –463 mg in the placebo group), with a sustained reduction in UACR (–18% versus –28% for placebo, P = 0.014). However, 2 μg paricalcitol was poorly tolerated.
In summary, when added to RAS blockade, VDR activators might have antiproteinuric effects in selected patients with T2DM. However, no evidence that these agents slow the progression of CKD exists to date.
As the patent for 1 μg paricalcitol capsules expired on 24 December 2013 and the patent for 2 μg paricalcitol capsules has expired on 24 June 201424 randomized controlled trials to investigate whether VDR activators preserve renal function are unlikely to be funded in the next few years.
Inhibitors of AGE formation
Advanced glycation end products (AGEs) accumulate in patients with diabetes25 and might activate the receptor for AGEs (RAGE), cause protein dysfunction and impair collagen turnover24. Pimagedine (also known as aminoguanidine) inhibited the formation of AGEs and slowed the progression of diabetic complications in animal models26.However a phase III randomized controlled trial in patients with T2DM was terminated because of adverse effects27 and the drug is no longer under development.
Pyridoxamine, the natural form of pyridoxine (vitamin B6), also prevents AGE formation. Two phase II randomized controlled trials have evaluated this agent in patients with T1DM or T2DM without affecting urinary albumin excretion the primary outcome of change in serum creatinine level from baseline at week 52.
ONGOING TRIALS
Endothelin-receptor antagonists
Endothelins are small vasoactive peptides with pleiotropic actions that contribute to hypertension, albuminuria, insulin resistance, inflammation, fibrosis and endothelial dysfunction28. The predominant isoform, endothelin-1, activates the endothelin-1 receptor (ETA) and the endothelin B (ETB) receptors ETB1 and ETB2. The ETA/ETB receptor antagonist bosentan prevented severe proteinuria and renal function impairment in experimental models of immune-complex nephritis29. The pathological effects of endothelin-1, including vaso-constriction, proteinuria, inflammation, cellular injury and fibrosis, are likely mediated by the ETA receptor, whereas ETB receptors generally exert a natriuretic effect28-31. Thus, use of ETA-selective drugs would not be expected to be associated with fluid retention. However, even very selective ETA blockers, such as sitaxsentan (ETA:ETB blockade 6,000:1) and avosentan cause fluid retention in patients to a similar degree as less-selective ETA-receptor blockers5,28-31. This finding is probably the result of effects of these agents on the proximal tubule, which lead to sodium retention by the kidney; however the mechanism has not been fully elucidated.
At present, the compound more advanced on clinical trials is atrasentan. This is a more-selective ETAreceptor antago-nist (ETA:ETB receptor selectivity 1,200:1) than avosentan. In a phase II randomized clinical trial, atrasentan in addition to RAS blockade significantly reduced UACR (by 42% and 35%, respectively) compared with placebo (reduction of 11%).
Peripheral oedema was observed in 18% patients in the low-dose atrasentan. The SONAR phase III randomized controlled trial is now assessing the effect of atrasentan versus placebo as adjuvant to RAS blockade in patients with T2DM, eGFR 25–75 ml/min/1.73 m2 and UACR 300–5,000 mg/g32. The primary outcome is time to the first occurrence of a composite renal end point (doubling of serum creatinine levels or the development of ESRD).
Mineralocorticoid receptor antagonists
The RAS component aldosterone is a steroid hormone that activates the mineralocorticoid receptor.
In addi-tion to a role in the regulation of sodium balance, aldosterone has direct pro-inflammatory and pro–fibrotic action33. MRAs are potent antiproteinuric agents in humans34.
Two phase II randomized clinical trials are currently investigating the antialbuminuric effects of the novel MRAs, MT-3995 and BAY 94-8862, as add-on therapy to RAS blockade in patients with diabetic kidney disease. However, the need to administer MRAs in the early phase of disease because of concerns regarding the risk of hyperkalaemia in patients with decreased renal function is likely to limit the investigation of their effects on hard end points and their regulatory approval for diabetic kidney disease.
Targeting inflammation
Chemokines secreted by stressed kidney cells activate receptors in inflammatory leukocytes and drive infiltration of leukocytes into the kidney35.
Targeting MCP-1, or its receptor CCR2, has been shown to reduce albuminuria and podocyte injury and increase eGFR in experi-mental models of diabetic kidney disease35-37. (Ongoing phase II randomized controlled trials are exploring the safety, tolerability and effect on albuminuria of the CCR2 antagonist CCX 140-B and the CCR2/5 antagonists PF-0463481738 and BMS-81316039).
Activation of Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signalling is another pathway for hyperglycaemia-induced kidney injury. Dysregulation of the JAK–STAT pathway has been documented in human progressive diabetic kidney disease.
Overexpression of the intracellular negative regulators of JAK–STAT signalling, suppressors of cytokine signalling (SOCS)-1 and SOCS-3, is also pro-tective in experimental diabetic kidney disease40. The oral JAK1 and JAK2 inhibitor baricitinib, which is being developed for rheumatoid arthritis, might also have renoprotective effects. An ongoing phase II randomized clinical trial is testing baricitinib as an adjuvant to RAS blockade in 250 patients with diabetic kidney disease and macroalbuminuria (UACR 300–5,000 mg/g). The primary outcome is change from baseline UACR at 24 weeks of treatment.
Other compounds
There are a number of experimental and clinical data suggesting that other compounds, such as xanthine oxidase inhibitors, anti-TGF-Beta antibodies and phosphodiesterase inhibitors, could have a role in the prevention and treatment of diabetic nephropathy.
In fact, small clinical trials are currently in progress to explore and extend the potential therapeutic involvement in those patients41.
CONCLUSIONS
Despite major advances in the knowledge of molecular and cell signalling pathways involved in kidney injury, few new drugs are coming into the market to treat diabetic kidney disease. Failure rates of new agents in randomized controlled trials are very high, exceeding 90% overall and 50% for phase III trials.
Several problems with testing new approaches to therapy in diabetic kidney disease impair the ability to identify therapeutic agents and might have contributed to the paucity of clinical advances in the past 10 years. Renal disease in T2DM might be heterogeneous both in terms of the underlying cause of kidney injury and of the stage of the disease. Since diabetic kidney disease is a clinical diagnosis, in the absence of a gold-standard diagnostic test (such as kidney biopsy), there is no cer-tainty that patients enrolled in diabetic kidney disease trials have the disease. Inclusion criteria in diabetic kidney disease trials vary widely from the presence of diabetes plus reduced GFR to diabetes and various ranges of eGFR, serum creatinine levels and albuminuria. The issue of disease heterogeneity is tightly linked to regulatory problems regarding acceptable end points. Given the difficulty of performing randomized controlled trials with hard end points, most studies focus on albuminuria as a first step towards taking a decision to go ahead with further studies. However, albuminuria and decline in renal function might be dissociated and studies targeting more robust end points, such as meas-ured GFR or progression to ESRD are sorely needed.
Only one ongoing study is aimed at achieving regulatory approval for a novel therapy (atrasentan) for diabetic kidney disease; however, safety concerns and the limited study population mean that this therapy is unlikely to repre-sent a breakthrough for the wider diabetic kidney disease population. We hope that the outstanding advances in knowledge of the molecular and cellular mechanisms involved in organ injury in diabetic patients will pave the way for the design of novel drugs and trials in the years to come. It is expected that, besides a better control of blood glucose, we will have a number of drugs to treat the complications of diabetes.
ACKNOWLEDGEMENTS
This work was supported by grants from the Spanish Ministry of Science (SAF 2012-38830 to C.G.-G.), Fondo de Investigaciones Sanitarias (FIS PS09/00447, FIS PI10/0072, PI14/00386, PI13/00047, FIS/PIE13/00051, ISCIII-RETIC REDinREN/RD06/0016 and 12/0021), Spanish Society of Nephrology, Comunidad de Madrid S2010/BMD-2378 and PRIORITY, as well as ISCIII Juan Rodes to B.F.-F., Programa Intensificacion Actividad Investigadora (ISCIII/Agencia Lain-Entralgo/CM) to A.O, and Diabetes kidney connect (Health-F2-2013-602422) and e-PREDICE (FP7-HEALTH-2011-279074) to to J.E.
References
1. Afkarian M, M. C. Sachs M C, B. Kestenbaum B, et al.. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013; 242:302-308.
2. Fernandez FB, Elewa U, M D Sanchez-Nino MD, et al.. 2012 update on diabetic kidney disease: the expanding spectrum, novel pathogenic insights and recent clinical trials. Minerva Med.2012; 1034:219-234.
3. Collins AJ, Foley RN, Chavers B et al.. US Renal Data System 2013 Annual Data Report. Am.JKidney Dis. 2014; 63 (1 Suppl):A7. [ Links ].
4. de Boer IH, Rue TC, Cleary PA, et al.. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med.2011; 1715:412-420. [ Links ].
5. Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003; 28924:3273-3277. [ Links ]
6. Molitch ME, Steffes M, Sun W, et al.. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010; 337:1536-1543. [ Links ]
7. Cushman WC, Evans GW, Byington RP, et al.. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N.Engl.J.Med. 2010; 36217:1575-1585. [ Links ]
8. KDIGO BP Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2012;2:337-414. [ Links ]
9. American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 2014;37(Suppl 1):S14-S80. [ Links ]
10. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49 (2 Suppl 2):S12-154. [ Links ]
11. Parving HH, Brenner BM, McMurray JJ, et al.. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 36723:2204-2213. [ Links ]
12. Fried LF, Emanuele N, Zhang JH et al.. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 36920:1892-1903. [ Links ]
13. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int 2013;3:259-305. [ Links ]
14. Martin JH, Deacon CF, Gorrell MD, J. B. Prins JB. Incretin-based therapies–review of the physiology, pharmacology and emerging clinical experience. Intern Med J 2011; 414:299-307.
15. Mikhail N. Place of sodium-glucose co-transporter type 2 inhibitors for treatment of type 2 diabetes. World J Diabetes 2014; 56:854-859. [ Links ]
16. Rossi MC, NicolucciA, Pellegrini F, et al. De. Obesity and changes in urine albumin/creatinine ratio in patients with type 2 diabetes: the DEMAND study. Nutr Metab Cardiovasc Dis 2010; 202:110-116. [ Links ]
17. Neff KJ, Frankel AH, Tam FW, Sadlier DM, Godson C, le Roux CW. The effect of bariatric surgery on renal function and disease: a focus on outcomes and inflammation. Nephrol Dial Transplant 2013; 28 Suppl 4:iv73-iv82. [ Links ]
18. Solano MP, Goldberg RB. Management of dyslipidemia in diabetes. Cardiol Rev 2006; 143:125-135. [ Links ] [ Links ]
20. de Zeeuw D., Akizawa T, Audhya P et al.. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013; 36926:2492-2503. [ Links ]
21. Sanchez-Nino MD, Bozic M, Cordoba-Lanus E, et al.. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol 2012; 302(6):F647-F657. [ Links ]
22. E. Gonzalez-Parra E, J. Rojas-Rivera J, J. Tunon J, M. Praga M, A. Ortiz A, Egido J. Vitamin D receptor activation and cardiovascular disease. Nephrol Dial Transplant 2012; 27 Suppl 4:iv17-iv21.
23. de Zeeuw D, Agarwal R, Amdahl M, et al.. Andress. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010; 3769752:1543-1551. [ Links ]
24. U.S. Department of Health and Human Services. Orange Book: approved drug products with therapeutic equivalence evaluations. US Food and Drug Administration [online], http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm2014 [ Links ]
25. Ramasamy R, Yan SF, Schmidt AM. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascul Pharmacol 2012; 57 (5-6):160-167. [ Links ].
26. Kim JH, Hong CO, Koo YC, Kim SJ, Lee KW. Oral administration of ethyl acetate-soluble portion of Terminalia chebula conferring protection from streptozotocin-induced diabetic mellitus and its complications. Biol Pharm Bul. 2011; 3411:1702-1709. [ Links ]
26. [No authors listed] Alteon may drop pimagedine in NIDDM. thepharmaletter [online], http://www.thepharmaletter.com/article/alteon-may-drop-pimagedine-in-niddm1998 [ Links ]
27. Kohan DE, Pollock DM. Endothelin antagonists for diabetic and non-diabetic chronic kidney disease. Br J Clin Pharmacol 2013; 764:573-579. [ Links ]
28. Gomez-Garre D, Largo R, Liu XH, Gutierrez S, Lopez-Armada MJ, Palacios I, Egido J. Na orally active ETA/ETB receptor antagonist ameliorates proteinuria and glomerular lesions in rats with proliferative nephritis. Kidney Int. 1996; 503:962-972. [ Links ]
29. Rodriguez-Vita J, M. Ruiz-Ortega M, Ruperez M, et al. Endothelin-1, via ETA receptor and independently of transforming growth factor-beta, increases the connective tissue growth factor in vascular smooth muscle cells. Circ Res 2005; 97(2):125-134..
30. Gomez-Garre D, Largo R, Tejera N, Fortes J, Manzarbeitia F, Egido J. Activation of NFkappaB in tubular epithelial cells of rats with intense proteinuria: role of angiotensin II and endothelin-1. Hypertension 2001; 374:1171-1178. [ Links ].
31. Andress DL, Coll B, Pritchett Y, Brennan J, Molitch M, Kohan DE. Clinical efficacy of the selective endothelin A receptor antagonist, atrasentan, in patients with diabetes and chronic kidney disease (CKD). Life Sci 2012; 91 (13-14):739-742. [ Links ]
32. Brem AS, Morris DJ, Gong R. Aldosterone-induced fibrosis in the kidney: questions and controversies. Am JKidney Dis 2011; 583:471-479. [ Links ].
33. Morales E, Millet VG, Rojas-Rivera J, et al. Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant 2013; 282:405-412. [ Links ].
34. Moreno JA, Moreno S, Rubio-Navarro A, et al.,. Targeting chemokines in proteinuriainduced renal disease. Expert Opin Ther Targets 2012; 168:833-845. [ Links ].
35. Sayyed SG, Ryu M, Kulkarni OP, et al.. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int 2011; 801:68-78. [ Links ],.
36. Sullivan T, Miao Z, Dairaghi DJ, et al.. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am J Physiol Renal Physiol 2013; 3059:F1288-F1297. [ Links ].
37. U.S. National Library of Medicine. ClinicalTrials.gov [online], http://www.clinicaltrials.gov/ct2/show/NCT017120612014. [ Links ]
38. US National Library of Medicine. ClinicalTrials.gov [online], http://www.clinicaltrials.gov/ct2/show/NCT017529852013. [ Links ]
39. Ortiz-Munoz G, Lopez-Parra V, Lopez-Franco O, et al.. Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol 20 10; 215:763-772. [ Links ]
40. Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, Egido J. Therapeutic approaches to diabetic nephropathy–beyond the RAS. Nat Rev Nephrol2014; 106:325-346. [ Links ]
Jesus Egido, M.D., Ph.D. Division of Nephrology and Hypertension,
Renal, Diabetes and Vascular Research Laboratory, IIS-Fundacion Jimenez Diaz.
Universidad Autonoma de Madrid, Avenida Reyes Catolicos 2.
28040 Madrid, Spain.
E-mail: ajegido@fjd.es
Conflict of interest statement: The authors declare no conflict of interests.
Received for publication: 20/01/2015
Accepted: 22/01/2015














