Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.2 Lisboa jun. 2016
REVIEW ARTICLE
Correcting acidosis in CKD patients.
How much is too much
Pedro Ponce
NephroCarePortugal
HOW TO DEAL WITH ACIDOSIS
Acid-base balance is normally maintained by the renal excretion of the daily acid load, about 1mEq/kg/day, derived mostly from the generation of sulphuric acid during the metabolism of dietary amino-acids. Elimination of this load is achieved by the urinary excretion of hydrogen ions, both as titratable acidity and predominantly as ammonia1. Total ammonium excretion begins to fall when the GFR drops below 40 to 50ml/min. At this level ammonium excretion per total GFR is three to four times above normal, meaning that it accommodates at the level of each remaining nephron the need for extra H+ excretion. The retained acid is buffered by bicarbonate (Bic) in the extracellular fluid, by tissue buffers and by bone. The anion gap remains normal until late stages of CKD, when it begins to widen due to the retention of anions such as phosphate, sulphate and urate.
In CKD, acidosis is generally mild to moderate, with a plasma Bic ranging from 12 to 22mEq/L, rarely below 12mEq/L in the absence of an increased exogenous acid load. Metabolic acidosis, therefore, results from the imbalance between the whole-body net production of acid, basically dependent on diet, and acid removal by the kidney.
ACIDOSIS AIN´T GOOD FOR YOU
In the non-CKD setting, the association between low serum bicarbonate (acidosis) and eGFR decline is well established2,3. In the Health ABC study, participants with a normal eGFR and a serum bicarbonate below 23mEq/L were at an increased risk of incident (de novo) CKD than were those with normal serum bicarbonate.
Regarding life expectancy, an analysis of the Third National Health and Nutrition Examination Survey (NHANES III) showed that participants with serum bicarbonate below 22mEq/L had a 76% higher mortality than that of those with normal serum bicarbonate, and this association was not modified by CKD status4. However in this study systemic pH and PCO2 were not measured, which is important as low serum bicarbonate in persons with preserved eGFR may not necessarily reflect primary metabolic acidosis as it does in CKD.
Subsequently, in a secondary analysis of the Health ABC study, a prospective study looking at well-functioning adults aged 70 to 79 years old, participants were grouped according to measurements of arterialized venous blood gases into below 23mEq/L (low), 23 to 28mEq/L (reference group) and higher than 28mEq/L (high) serum bicarbonate categories, with a mean follow-up of 10.3 years. Compared to the reference group, the hazard ratios for mortality were 1.24 for the low serum bicarbonate group, 1.03 for the high serum bicarbonate, 1.17 for the metabolic acidosis group, 1.21 for those with respiratory alkalosis and 1.35 for the metabolic alkalosis group, meaning that low serum bicarbonate was associated with higher mortality, independent of systemic pH and potential confounders5.
Covesdy and colleagues, in a study including 1,240 military veterans with non-dialysis dependent CKD, showed that those with a serum bicarbonate below 22mEq/L were at a significantly increased risk of mortality than those with a serum bicarbonate of 26 to 29mEq/L (adjusted hazard ratio 1.43)6.
Metabolic acidosis is associated with several negative consequences7, including tubulointerstitial fibrosis and kidney damage8, bone demineralization9, increased protein catabolism and inflammation, sarcopenia10, stimulation of the renin-angiotensin system and of adrenocorticotrophic hormone11, impaired glucose tolerance, insulin resistance and resistance to growth hormone and IGF, and accumulation of β2 microglobulin7.
Correction of metabolic acidosis with alkali seems to correct many of these effects12, although it is uncertain whether it improves survival.
Results from several studies suggest that raising low serum bicarbonate may improve clinical outcomes in persons with preserved GFR. However the results also caution against raising serum bicarbonate too high as this might increase the mortality risk from metabolic alkalosis. Targeting a serum bicarbonate concentration of 24 to 26mEq/L seems reasonable. Chronic metabolic alkalosis may increase mortality also by inducing vascular calcification13.
BICARBONATE USE FOR PREVENTION OF CKD PROGRESSION.
As stated above, metabolic acidosis, which commonly accompanies CKD, results from several mechanisms, including decreased nephron mass (which reduces overall ammonia production although increasing its production at each remaining nephron), decreased proton secretion at the tubular level, and hyperkalaemia (which suppresses ammoniagenesis).
Metabolic acidosis usually occurs in the early stages of CKD as an acidosis with a normal anion gap, and then, as CKD progresses to the advanced stages, patients present with an elevated anion gap as a result of organic anions accumulation.
Metabolic acidosis promotes an adaptive increase in ammonium excreted per nephron, which is associated with activation of the complement system and of the renin-angiotensin system, and with increased renal production of endothelin 1, and these changes may produce tubulointerstitial inflammation and chronic damage to the kidney14. However, and in spite of the persistently positive proton balance, pH and serum bicarbonate remain stable as bone is also involved in buffering during the chronic stages of metabolic acidosis15.
Subclinical acidosis may be present in CKD, usually with normal serum bicarbonate and clinically unapparent acidosis, and it is associated with compensatory ammoniagenesis and detrimental increases in aldosterone and endothelin16,17.
Abramowitz and colleagues, in a single-blinded pilot study including 20 adult patients with an eGFR of 15 to 45mL/min per 1.73 m2 and a serum bicarbonate of 20 to 24mEq/L, evaluated the effect of treating during successive 2-week periods with placebo and escalating doses of oral bicarbonate on handgrip strength and on a sit-to-stand test (time required to complete 5 and 10 repetitions). The study showed that correction of acidosis by oral bicarbonate administration, leading to an increase in serum bicarbonate level in the range of 23 to 24mEq/L, had no effect on handgrip strength and was associated with an improvement in the sit-to-stand time. After 6 weeks of oral bicarbonate, urinary nitrogen excretion decreased. These results were interpreted as oral bicarbonate supplementation reduces protein catabolism and improves nutritional status and muscle function18.
In a post hoc analysis of the African American Study of Kidney Disease and Hypertension (AASK) trial, among 1,094 patients with CKD participating in the study, the renal outcome, which was a composite outcome, defined as the occurrence of end-stage renal failure, a 50% decline in GFR, or a reduction in GFR from baseline of at least 25ml/min, was approximately three times higher in patients whose serum bicarbonate was lower than 20mEq/L as compared with those whose serum bicarbonate was greater than 25mEq/L19. After controlling for possible confounders, a serum bicarbonate of 28 to 30mEq/L was associated with the lowest risk for the renal outcome.
Data from a retrospective cohort study which included 5,422 CKD patients visiting a medical clinic in the Bronx, NY from January 2001 to December 2003 and followed until June 2007, suggested that those patients with a serum bicarbonate of 22mEq/L or lower, as compared with those patients in the reference group (bicarbonate level 25 to 26mEq/L), were at a significantly increased risk of progression of renal disease (adjusted hazard ratio 1.54; 95%CI 1.13 to 2.09)20. In this study, kidney disease progression was defined as either a decline in eGFR by 50% or reaching an eGFR of <15mL/min/1.73m2.
The treatment of metabolic acidosis usually consists of administration of sodium bicarbonate or of sodium citrate, typically in a dose of 0.5 to 1mEq/Kg/day. Sodium citrate should not be administered in patients taking aluminium-based phosphate binders, as it dramatically enhances intestinal aluminium absorption. Alternative treatments are calcium acetate and calcium carbonate, which are also frequently used as phosphate binders.
Noteworthy, although sodium chloride and sodium bicarbonate represent identical sodium loads to the body, the impact of their use on blood pressure values and extracellular volume seems to be distinct. For example, in dialysis patients oral bicarbonate is not associated with an increase in inter-dialytic weight gain21,22.
For practical purposes, 1gram of sodium bicarbonate has 12mEq of sodium and of bicarbonate, and 1mEq of bicarbonate has 84mg of the salt (sodium bicarbonate).
Tolerance to the oral presentations available (baking soda, Alka-Seltzer©) is poor, and thus there is usually a low rate of adherence to this therapy, making recourse to other options, such as sodium carbonate or citrate, often necessary.
The best data to suggest that bicarbonate supplementation may slow down the progression of CKD comes from a single-centre trial which included 134 stage 4 CKD patients with metabolic acidosis (Bic 16 to 20mEq/L). Patients were randomly assigned to either supplementation with oral sodium bicarbonate (600mg thrice daily increased as necessary to achieve and maintain a serum bicarbonate level equal to or greater than 23mmol/L) or standard of care for a 2-year period.
This was an open-label, randomised, prospective, parallel-group trial. The principal investigator who enrolled the patients was blind to group allocations until the end of the study. The primary endpoints were decline in renal function (estimated by measured creatinine clearance) over the follow-up period, number of patients with a fall in creatinine clearance equal to or greater than 3mL/min per year, and ESRD. The study showed that, compared to the control group, patients receiving sodium bicarbonate supplementation had a lower decline in creatinine clearance over the 2-year period (1.88 versus 5.93 mL/min; p<0.0001); were less likely to experience a fall in creatinine clearance equal to or greater than 3mL/min per year (9 versus 45%; relative risk 0.15; 95%CI 0.06 to 0.40), and fewer developed ESRD (6.5 versus 33%; relative risk 0.13 95%CI 0.04 to 0.40). However, this was an open-label trial, and thus with a high risk of bias12.
Similar results were observed by Mahajan and colleagues23.
The study included 120 patients with hypertensive nephropathy, with an average eGFR of 75±6 ml/min/1.73m2 and a serum bicarbonate of at least 24mEq/L, randomly allocated to receive oral bicarbonate 0.5mEq/kg/day (40 patients), oral sodium chloride 0.5mEq/Kg/day (40 patients), or placebo (40 patients), and were followed for a 5-year period21. The primary endpoint was the rate of eGFR decline. Patients who received bicarbonate experienced significantly more favourable slopes of eGFR decline compared with both the placebo and the sodium chloride groups. Additionally, patients who received bicarbonate showed a decrease in urinary markers of tubular injury, a reduction of urine endothelin, and stabilisation of albuminuria, suggesting that renoprotective interventions with oral alkali therapy could be applied preemptively at stages when renal adaptive mechanisms are still capable of maintaining the body´s acid-base balance.
Unfortunately all these trials are single-centre in nature, of small size, and use different therapeutic approaches, and it is therefore hard to generalise their findings.
In conclusion, it is recommended, until further evidence becomes available, that in CKD clinic, patients are regularly monitored with total CO2 (alkaline reserve) determinations and treated to a goal of 24 to 26mEq/L, with the most tolerable form of oral alkalinisation.~
ACIDOSIS CORRECTION IN HAEMODIALYSIS PATIENTS
In the time between dialysis treatments, serum bicarbonate gradually decreases because of both the production of endogenous acids (mostly coming from diet) and the retention of fluid. In contrast to individuals with normal renal function, no homeostatic feedback loop exists to link acid production to acid excretion and maintain acid-base equilibrium24,48.
The correction of acidosis in end-stage renal failure patients on dialysis is achieved by the transfer of sodium bicarbonate present in high concentration (32 to 38mEq/L) in the dialysate solution to the blood compartment, following a steep concentration gradient.
Bicarbonate does not traverse the cell membrane; therefore its intracellular concentration remains stable at around 10mEq/L.
The acid concentrate (that contains calcium chloride), is mixed by the dialysis machine with the base concentrate (mostly sodium bicarbonate) and water to obtain the final dialysate solution. This acid concentrate needs an organic acid in its composition, most commonly acetic acid / sodium diacetate 4 to 8mmol/L, to avoid, once mixed, the insoluble precipitation of calcium carbonate. This acetate anion will be metabolized to bicarbonate, increasing the total amount of buffer in the final dialysate25. In many haemodialysis units there is an unrecognised use of a high dialysate buffer due to a lack of awareness that the small amount of acetate (usually 6mmol/L) which is usually present in dialysate, is metabolised and generates additional bicarbonate in the body46.
With a bicarbonate dialysate set around 35mmol/L, the average predialysis serum bicarbonate is highly variable, ranging from less than 17 to more than 27mmol/L.
Bicarbonate typically increases rapidly during the first 2 hours of treatment to subsequently level off and, by the end the session, serum bicarbonate is about 4 to 7mmol/L below the dialysate bicarbonate26,27.
The reason why post-dialysis serum bicarbonate is below dialysate bicarbonate level, and not at the same level as we would expect for a small molecule, likely results from stimulation of organic acid production associated with bicarbonate transfer from dialysate to plasma, thus minimising further increase in serum bicarbonate26. Gennari and colleagues showed more than 20 years ago that the acidaemia of dialysis patients was relatively resistant to correction by treatment with a high dialysate bicarbonate, despite the fact that the amount of bicarbonate being transferred from dialysate to plasma was estimated as being far in excess of what was needed to do so. He postulated that an alkalosisinduced increase in acid generation was responsible; by minimising intra-dialytic alkalosis, less acid would be generated and, therefore, the amount of bicarbonate that would need to be transferred to plasma to correct acidosis would be reduced48. It is likely that acidosis can be fully corrected if only limited intradialytic alkalosis is induced by dialysis, which will probably need some form of variable dialysate bicarbonate concentration profiling along the course of dialysis treatment49.
Along the course of dialysis treatment there is an unexpected continuous decay of bicarbonate dialysance, and irrespective of the modality used, approximately the same acid-base parameters are achieved at the end of the treatment, whether through regular haemodialysis, high-flux haemodialysis, or online postdilution haemodiafiltration28. Thus bicarbonate dialysance behaves very differently from urea clearance, even though both solutes have a similar molecular weight28.
Applying the pharmacological concept of volume of distribution, a distribution volume for bicarbonate, or apparent bicarbonate space, is defined as the ratio of administered bicarbonate to the observed change in the plasma bicarbonate concentration. The great problem when dealing with bicarbonate kinetics is to calculate the apparent bicarbonate distribution space and bicarbonate mass (Bic space x Bic concentration) at the beginning and at the end of the dialysis session.
Blood pH seems to influence the bicarbonate space. In an experimental study in dogs, apparent bicarbonate space was 88% of body weight in acidotic animals, and 50% and 44% in normal and alkalotic dogs, respectively, showing an inverse correlation between the initial plasma bicarbonate concentration and the apparent bicarbonate space. This data confirmed the already known expansion and contraction of the ABS in metabolic acidosis and in metabolic alkalosis, respectively29.
Some studies also suggest that there is a positive relationship between the inter-dialytic weight gain and the apparent bicarbonate space, as the greater the interdialytic weight (water) gain, the higher the dialysate bicarbonate concentration required to achieve a normal acid-base status30. Administered bicarbonate during dialysis reacts with plasma and extra-plasma acidic buffers with an estimated space of distribution of 45 and 60%, after 30 and 90 minutes, respectively.
In summary, much more sophisticated computational models are needed to help us determine the optimal dialysate bicarbonate.
In this context, where evidence is mostly based on registries or retrospective studies, the K-DOQI guidelines recommend adjusting dialysate bicarbonate to achieve a pre-HD serum bicarbonate of at least 22mEq/L36 and the European Best Practice Guidelines recommend values between 20 to 22mEq/L37, but the issue is far from being consensual.
Lefebvre and colleagues, in a small study, randomly allocated haemodialysis patients to two therapeutic groups: 10 patients (group A) were dialysed with the conventional concentration of bicarbonate (33mmol/L) in dialysate, and 11 patients (group B) had 7 to 15 mmol/L sodium bicarbonate added to the dialysate to achieve a predialysis plasma bicarbonate of 24mEq/L. Group A achieved a pre-HD serum bicarbonate of 15.6mEq/L. Compared to the baseline, bone histology and hyperparathyroidism improved in group B but not in group A on the biopsies performed 18 months after the start of the study40. Movilli and colleagues, in a small study including 12 patients on haemodialysis, evaluated the effects of 3 months of correction of metabolic acidosis by oral bicarbonate supplementation on protein catabolic rate (PCR) and serum albumin concentration. The study had no control group. Predialysis serum bicarbonate increased from 19.3±0.6mmol/L to 24.4±1.2mmolL. Serum albumin increased from 3.5±0.2 to 3.8±0.3g/dL (p<0.01), and PCR decreased from 1.1±0.2 to 1.0±0.2g/kg per day (p<0.001). Authors conclude that, in haemodialysis patients, correction of metabolic acidosis improves serum albumin concentration and reverses muscle wasting and weakness41. Higher dialysate bicarbonate concentration and base administration improve protein turnover, nutritional status and the severity of mineral bone disease12,42,43 while, on the other hand, there was a suggestion that alkalosis may favour vascular calcification44.
A number of recent epidemiologic studies have suggested that higher bicarbonate dialysate concentrations or a higher level of achieved serum bicarbonate may counterintuitively be associated with an increased risk of death31,32,33. These paradoxical associations may be another example of reverse epidemiology and stem from the confounding role of better nutritional status and higher dietary protein intake, which result in acidaemia, but also in improved survival. After standardizing data for comorbidity and the malnutrition-inflammation complex, uncorrected acidosis is associated with worse outcomes, with adjusted survival rates reduced when predialysis bicarbonate is below 18mEq/L, as well as when it is greater than 24mEq/L33,34,45. Thus, our goal should be to seek the benefits of correcting acidosis in HD patients, while avoiding the risks of intra-dialytic alkalosis.
In 1990, Lowrie and colleagues reported in a retrospective analysis of 12,000 patients a U-shaped relationship between baseline serum bicarbonate and allcause mortality, with a higher risk of death when serum bicarbonate was below 17.5 or was greater than 25mEq/L31. The same U-shaped association between mortality and serum bicarbonate levels was reported by Bommer and colleagues in a multivariate analysis based on 7,000 patients assessed in the DOPPS study, with worse outcomes when serum bicarbonate was below 17mEq/L, or was greater than 27mEq/L, and a mild pre-HD acidosis (20 to 21mEq/L) was associated with better nutritional status and lower mortality. However, after adjustment for comorbidities, nutritional factors and dialysis adequacy, the apparent association between metabolic alkalosis and mortality became non-significant33. Also in a landmark study, using prospectively a database of 56,000 US haemodialysis patients followed from 2001 to 2003, in an unadjusted analysis, patients who had an acidotic range of serum bicarbonate (17 to 22mEq/L) exhibited better survival, while patients who had a normal to alkalotic range of serum bicarbonate (23 to 27mEq/L) showed an increase in all-cause and cardiovascular mortality, but these associations reversed almost entirely after multivariate adjustments for case-mix and for markers of nutritional status and inflammation34.
Wu and colleagues, in a study including 56,385 haemodialysis patients, found the 2-year mortality rate was lowest among those whose serum bicarbonate was in the range of 17 to 19mEq/L; however normal bicarbonate concentrations in dialysis patients usually reflect reduced protein intake and malnutrition, so after controlling their data for markers of poor nutrition, the authors found that a serum bicarbonate below 22mEq/L is associated with an increased mortality risk as compared with patients with higher serum bicarbonate values35. Thus the apparent pre-dialysis alkalaemia was likely an indicator of poor protein intake from the underlying anorexia and infers a poor prognosis.
In the opposite direction Vashista and colleagues analysed a national cohort of more than 160,000 haemodialysis patients between 2001 and 2007, and found that 40% of the patients had inadequate correction of metabolic acidosis, with a pre-dialysis serum bicarbonate below 22mEq/L. After adjusting for case-mix and nutritional and inflammatory markers, a pre-dialysis bicarbonate level below 24mEq/L was associated with a higher risk of death irrespective of dialysis modality, while a serum bicarbonate concentration greater than 25mEq/L was not associated with increased risk of death50.
The Nephrocare-Portugal Montijo group (Viegas M et al, personal communication) conducted a 6-month prospective trial involving 93 patients treated in-centre with online haemodiafiltration treatment, comparing a dialysate bicarbonate concentration of 30 versus 34mEq/L (to be added to 6 mmol/L of acetate in the acid component of the dialysate). Average pre-and post-dialysis serum bicarbonate were 21.1 and 25.3mEq/L, respectively, when dialysate concentration was 30mEq/L, and 22.7 and 28.0 mEq/L, respectively, when dialysate concentration was 34mEq/L. Post-dialysis, 51% of patients on the higher dialysate bicarbonate concentration had alkalosis, while none in the lower dialysate bicarbonate concentration had alkalosis. Following these results, the authors adopted the lower dialysate bicarbonate concentration as the standard concentration in the Unit.
We recently conducted a cross-section survey, in another of our NephroCare-Portugal Units, measuring arterialised blood gases plus electrolytes at time 0, 15, 30, 60 and 240 minutes of dialysis treatment time, in 186 patients treated by online haemodiafiltration (olHDF), using a dialysate bicarbonate concentration of 32 + 6 mEq/L and a potassium concentration (K) of 2mEq/L.
Figure 1 shows the average intra-dialytic progression of serum bicarbonate, pH and potassium, and Figure 2 shows the distribution of serum bicarbonate values pre-and post-dialysis.
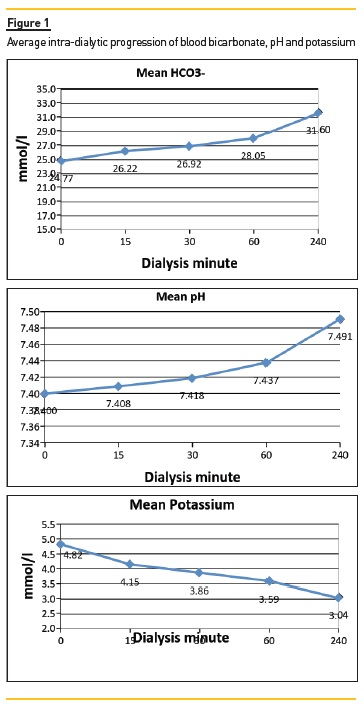
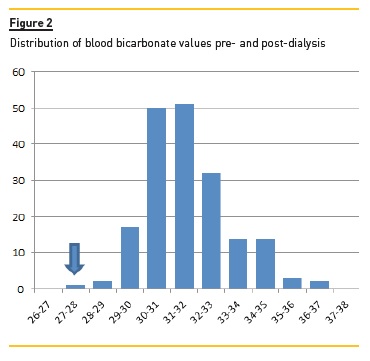
We confirmed a wide variation of pre- and postdialysis acid-base status, with many patients already alkalotic and hypokalaemic at the beginning of dialysis.
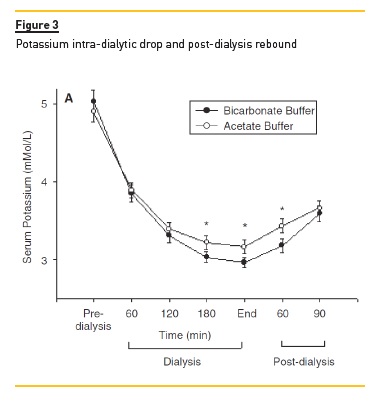
A close association between higher serum bicarbonate levels and lower serum K levels was also noted, with a quick rebound of serum K within the first hours post-treatment, as seen in Figure 3. We did not find any relationship between these findings and any consistent clinical manifestations (internal unpublished data).
In November 2011, Fresenius M.C. North America sent an internal memo to all its units, stating that the total buffer that patients were receiving could be underestimated, and that the average pre-dialysis serum bicarbonate rose over time from 22.9mEq/L in 2004 to 24.1mEq/L in 2011, with 15% of all patients above 28mEq/L, and that an internal analysis indicated an unadjusted odds ratio for cardiopulmonary arrest of 6.3 with pre-dialysis serum bicarbonate above 28mEq/L, recommending a reduction in dialysate bicarbonate concentration whenever pre-dialysis serum bicarbonate was higher than 24mEq/L.
Two recent studies concurred with this concern. In the first study, Tentori and colleagues, using DOPPS data, published an international prospective cohort study in 17,031 patients from 11 countries, and showed a positive association between dialysate bicarbonate concentration and all-cause mortality, with a 9% higher mortality risk per each 4 mEq/L increase in dialysate bicarbonate51. The second study, an observational study from the Japanese Society of Dialysis Therapy, enrolling 15,132 patients, found that a pH greater than 7.40 was the only pre-dialysis factor associated with a significantly increased risk of all-cause and cardiovascular mortality52. A question raised by the authors was whether the distinct mortality rates observed among countries could be due to differences in the usual dialysate bicarbonate concentration used (around 38mEq/L in the US, 34mEq/L in Europe and 30mEq/L in Japan)53.
The main adverse effects associated with a high dialysate bicarbonate concentration in patients treated by haemodialysis are the following53: a) Excess generation of CO2, requiring an increase in minute ventilation, hard to cope with in chronic respiratory failure patients; b) Fall in ionised calcium, and a drop in serum potassium both by removal to the dialysate and by rapid the shift from extracellular to the intracellular space associated with the fast correction of acidosis; c) Arrhythmias, as a sudden increase in serum bicarbonate and a drop in serum K and ionised calcium induces a QT-interval prolongation, which persists long-time after the end of dialysis session; d) Haemodynamic instability, as the electrolyte changes described earlier may cause a decrease in peripheral vascular resistance and the occurrence of hypotension38,39,47; e) Calcium phosphate precipitation in the vascular wall.
In conclusion, metabolic alkalosis is probably as harmful as metabolic acidosis, but before embarking on manipulating dialysate bicarbonate concentration we must know whether the level of pH and serum bicarbonate are the causes of morbidity and mortality or simply a marker of other factors responsible for those outcomes. It is suggested that the ideal level for predialysis serum bicarbonate is somewhere between 18 and 23mEq/L, which typically represents a metabolic acidosis, although we must be aware that pre-dialysis determinations are the nadir value for each patient.
We must pay special attention to values of serum bicarbonate below 18mEq/l or above 27mEq/L.
As final recommendations, which are mostly opinionbased, we suggest that for patients with low pre-dialysis serum bicarbonate values, we must ensure that alkali was indeed delivered, measuring post-dialysis serum bicarbonate. If serum bicarbonate increased normally during the treatment, patient´s diet and fluid intake should be assessed as excess protein intake or fluid retention are probably the cause of low pre-dialysis serum bicarbonate, and, if this is the case, only if we cannot reverse this trend should we then increase dialysate bicarbonate concentration. On the other hand, for patients with high pre-dialysis serum bicarbonate, the first priority should be to correct nutritional deficiencies, which is usually more effective than reducing dialysate bicarbonate concentration26,35,54.
References
1. Bailey J. Metabolic acidosis: an unrecognized cause of morbidity in the patient with chronic kidney disease. Kidney Int. 2005; 68(Suppl 96): S15-S23 2 - Driver T, [ Links ] Shlipak M, Katz R, et al. Low serum bicarbonate and kidney function decline: the MESA study. Am J Kidney Dis. 2014; 64:534-541 [ Links ]
3. Goldenstein L, Driver T, Fried L, et al. Serum bicarbonate concentration and kidney disease progression in community-living elders: the Health ABC study. Am J Kidney Dis. 2014; 64:542-549 [ Links ]
4. Raphael K, Zhang Y, Wei G, et al. Serum bicarbonate and mortality in adults in NHANES III. Nephrol Dial Transplant. 2013; 28:1207-1213 [ Links ]
5. Raphael K, Murphy R, Shlipak M, et al. Bicarbonate concentration, acid-base status, and mortality in the health, aging, and body composition study. Clin J Am Soc Nephrol. 2016; 11:308-316 [ Links ]
6. Kovesdy C, Anderson J, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis dependent CKD. Nephrol Dial Transplant. 2009; 24:1232-1239 [ Links ]
7. Kopple J, Klantar-Zadeh K, Mehrotra R. Risk of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. 2005; 67:521-527 [ Links ]
8. Wesson D, Simoni J. Increase tissue acid mediates a progressive decline in glomerular filtration rate on animals with reduced nephron mass. Kidney Int. 2009; 75:929-935 [ Links ]
9. Bushinsky D, Chabala J, Gavrilov K, et al. Effects of in vivo metabolic acidosis on midcortical bone ion composition. Am J Physiol. 1999; 277:F813-F819 [ Links ]
10. Kalantar-Zadeh K, Mehrotra R, Fouque D, et al. Metabolic acidosis and malnutritioninflammation complex syndrome in chronic renal failure. Semin Dial. 2004; 17:455-465 [ Links ]
11. Ng H, Chen H, Tsai Y, et al. Am J Nephrol. 2011; 34:55-63 [ Links ]
12. Ashurst I, Varagunam M, Raftery M, et al. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009; 20:2075-2084 [ Links ]
13. Solis A, Pacheco F, Deudero J, et al. Alkalinization potentiates vascular calcification deposition in an uremic milieu. J Nephrol. 2009; 22:647-653 [ Links ]
14. Green J, Kleeman C. Role of bone in regulation of systemic acid-base balance. Kidney Int. 1991; 39:9-26 [ Links ]
15. Wesson D. Endogenous endothelins mediate increased distal tubule acidification induced by dietary acid in rats. J Clin Invest. 1997; 99:2203-2211 [ Links ]
16. Frasseto L, Hsu C. Metabolic acidosis and progression of chronic kidney disease. J Am Soc Nephrol. 2009; 20:1869–1878 [ Links ]
17. Wesson D, Simoni J. Increased tissue acid mediates a progressive decline in glomerular filtration rate of animals with reduced nephron mass. Kidney Int. 2009; 75:929-935 [ Links ]
18. Abramowitz M, Bauer C, Raff A, et al. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol. 2013; 8:714-720 [ Links ]
19. Raphael K, Wei G, Baird B, et al. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011; 79:356-364 [ Links ]
20. Sha S, Abramowitz M, Hostetter T, et al. Serum bicarbonate levels and the progression of kidney disease. Am J Kidney Dis. 2009; 54:270–278 [ Links ]
21. Kurtz T, Al-Bander H, Morris R. Salt-sensitive essential hypertension in men. Is the sodium ion alone important? N Eng J Med. 1987; 317:1043-1048 [ Links ]
22. Movilli E, Gaggia P, Camerini C, et al. Effect of oral sodium bicarbonate supplementationon interdialytic weight gain, plasma sodium concentration, and predialysis blood pressure in hemodialysis patients. Blood Purif. 2005; 23:379-383 [ Links ]
23. Mahajan A, Simoni J, Sheather S, et al. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010; 78:303-309 [ Links ]
24. Gennari F. Acid-base balance in dialysis patients. Semin Dial. 2000; 13:235-239 [ Links ]
25. Sam R, Vaseemuddin M, Leong W, et al. Composition and clinical use of hemodialysates. Hemodial Int. 2006; 10:15-28 [ Links ]
26. Gennari F. Very low and high predialysis serum bicarbonate levels are risk factors for mortality. Semin Dial. 2010; 23:253-257 [ Links ]
27. Basile C, Libutti P, DiTuro L, et al. Effect of dialysate calciun concentration on parathormone and calcium balanceduring a single dialysis session using bicarbonate hemodialysis. Am J Kidney Dis. 2012; 59:92-101 [ Links ]
28. Morel H, Jaffrin M, Lux C, et al. A comparison of bicarbonate kinetics and acid-base status in high-flux hemodialysis, and online post-dilution hemodiafiltration. Int J Artif Organs. 2012; 35:288-300 [ Links ]
29. Garella S, Dana C, Chazan J. Severity of metabolic acidosis as a determinant of bicarbonate requirements. N Eng J Med. 1973; 289:121-126 [ Links ]
30. Tzanatos H, Dalamangas A, Retsa K, et al. Relation of interdialytic water retention with apparent bicarbonate space and pH in hemodialysed uremic patients. Renal Fail. 2005; 27:235-238 [ Links ]
31. Lowrie E, Lew N. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990; 15:458-482 [ Links ]
32. Tentori F, Robinson B, Morgensten H, et al. Association of dialysate bicarbonate concentration with mortality in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2013; 62:738-746 [ Links ]
33. Bommer J, Satayathum S, Keen M, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the dialysis outcome and practice patterns study (DOPPS). Am J Kidney Dis. 2004; 44:661-671 [ Links ]
35. Wu D, Shinaberger C, Regidor D, et al. Association between serum bicarbonate and death in hemodialysis patients. Clin J Am Soc Nephrol.2006; 1: 70-78 [ Links ]
36. National Kidney Foundation. K-DOQI Guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003; 42(Suppl 3): S1-S202 [ Links ]
37. Fouque D, Vennegoor M, Wee P, et al. EBPG on nutrition. Nephrol Dial Transplant. 2007; 22:ii45-ii87 [ Links ]
38. DiLorio B, Torraca S, Piscopo C, et al. Dialysate bath and QTc interval in patients on chronic maintenance hemodialysis. J Nephrol. 2012; 25:653-660 [ Links ]
39. Gabutti L, Ferrari N, Giudici G, et al. Unexpected hemodynamic instability associated with standard bicarbonate hemodialysis. Nephrol Dial Transplant. 2003; 18:2369-2376 [ Links ]
40. Lefebvre A, Vernejoul M, Gueris J, et al. Optimal correction of acidosis changes progression of dialysis osteodistrophy. Kidney Int. 1989; 36:1112-1119 [ Links ]
41. Movilli E, Zani R, Carli O, et al. Correction of metabolic acidosis increases serum albumin concentrations and decreases kinetically evaluated protein intake in hemodialysis patients. Nephrol Dial Transplant. 1998; 13:1719-1725 [ Links ]
42. Verove C, Maisonneuve N, Azouzi A, et al. Effect of the correction of metabolic acidosis on nutritional status in elderly patients with chronic renal failure. J Ren Nutr. 2002; 12:224-228 [ Links ]
43. Mathur R, Dash S, Gupta N, et al. Effects of correction of metabolic acidosis on blood urea, and bone metabolism in patients with mild to moderate chronic kidney disease. Ren Fail. 2006; 28:1-5 [ Links ]
44. Goodman W, London G. Vascular calcification in chronica kidney disease. Am J Kidney Dis. 2004; 43:572-579 [ Links ]
45. Kovesdy C, Anderson J, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. 2009; 24:1232-1237 [ Links ]
46. Kohn O, Kjellstrand C, Ing T. Dual-concentrate bicarbonate-based hemodialysis: Know your buffers. Artif Organs. 2012; 36:765-768 [ Links ]
47. Gabutti L, Bianchi G, Soldini D, et al. Hemodynamic consequences of changing bicarbonate and calcium concentrations in hemodialysis fluids. Nephrol Dial Transplant. 2009; 24:973-981 [ Links ]
48. Gennari G. Acid-base homeostasis in end-stage renal disease. Semin Dial. 1996; 9:404-411 [ Links ]
49. Tovbin D, Sherman R. Correcting acidosis during hemodialysis: Current limitations and a potential solution. Semin Dial. 2016; 29:35-38 [ Links ]
50. Vashista T, Molnar M, Torlen K, et al. Dialysis modality and correction of uremic metabolic acidosis. Clin J Am Soc Nephrol. 2013; 8:254-264 [ Links ]
51. Tentori F, Karaboyas A, Robinson B, et al. Association of dialysate bicarbonate concentration with mortality in the DOPPS study. Am J Kidney Dis. 2013; 62:738-746 [ Links ]
52. Yamamoto T, Shoji S, Yamakawa T, et al. Predialysis and postdialysis pH and bicarbonate and risk of all-cause mortality in long-term hemodialysis patients. Am J Kidney Dis. 2015; 66:469-478 [ Links ]
53. Basile C, Rossi L, Lomonte C. The choice of dialysate bicarbonate: do different concentrations make a difference? Kidney Int. 2016; 89:1008-1015 [ Links ]
54. Bommer J, Locatelli F, Satayathum S, et al. Association of the predialysis serum bicarbonate levels and the risk of mortality and hospitalization in the DOPPS. Am J Kidney Dis. 2004; 44:661-671 [ Links ]
Pedro Ponce, MD
NephroCare Portugal
E-mail: pedro.ponce@fmc-ag.com
Disclosure of potential conflicts of interest: The author is an employee of Fresenius Medical Care
Received for publication: May 27, 2016
Accepted in revised form: Jun 10, 2016














