Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.3 Lisboa Sept. 2016
ORIGINAL ARTICLE
Aldosterone levels in patients on haemodialysis: Relationship with body fat mass and adipocytokines
MJ Fernández-Reyes1, M Heras1, MJ Gonzalez Villalba2, O. Rodriguez Fraga2, A. Molina1, R. Callejas1, A. Rodríguez1, V. Lopes3, L. Calle1
1 Nephrology Department. Complejo Asistencial de Segovia.
2 Clinical Laboratory Department. Hospital Universitario la Paz.
3 Centre of Dialysis Los Olmos FRIAT. Segovia
ABSTRACT
Background: It has been recently shown that serum aldosterone (SA) levels are correlated with insulin resistance, excess body fat and levels of adipocytokines. Aim: To establish SA levels in patients on haemodialysis (HD) and the possible association with excess body fat and/or serum adipocytokine levels. Methods: 28 stable patients on HD. Mean age: 73.7±13.3 years; 53.6% men. Mean time on HD: 40.2± 40.8 months. None were diabetics nor treated with drugs that interfere with the renin-angiotensin-aldosterone-system. 18 patients were anuric. All measurements were performed prior to the midweek HD session. Results: SA levels (median: 28.1; 25th percentile (p25):10.4; 75th percentile (p75):98.6 ng/dl) were above the normal range in 53.6% of patients. Plasma renin activity (PRA) (median: 1.05; p25: 0.16; p75: 3.1 ng/mL/hour) was above the normal range in 21.4% of patients. There were no statistically significant differences in SA levels between anuric and non-anuric, male and female, presence and absence of myocardiopathy. There were no statistically significant correlations of SA levels or PRA with urine volume, residual renal function, dose or time on dialysis. age, or comorbidity. SA levels were positively correlated with PRA (r=000.70; p< 0.0001); body fat mass (r=0.40; p=0.03); leptin (r=0.45; p =0.01); leptin/adiponectin ratio (r=0.52 p=0.005) and negatively with serum adiponectin levels (r=- 0.37; p=0.05). In stepwise multiple regression analysis, the best model to explain SA levels included PRA and LAR (r=0.78; r2=0.60). Conclusions: SA levels were elevated in a high percentage of HD patients. SA levels were correlated with PRA, body fat mass and adipocytokines.
Key-words: Aldosterone, haemodialysis, adiponectin, leptin, boy mass fat.
INTRODUCTION
Cardiovascular (CV) disease is the major cause of morbidity and mortality in patients with chronic kidney disease (CKD) Stage 5, and its prevalence is markedly greater than in the age-matched general population.
Such increased risk is due to the high prevalence of traditional and kidney-related risk factors1. The role of adipose tissue and adipocytokines in CV risk in this population is not sufficiently defined. In contrast to the general population, a higher body mass index (BMI) is associated with better survival among haemodialysis (HD) patients2, although an excess of body fat mass is associated with insulin resistance in non-diabetic chronic HD patients3. Altered secretory patterns of adipose tissue may play a crucial role in the metabolic disturbances observed in advanced kidney disease and have been hypothesised to play a role in insulin resistance.
Leptin and adiponectin play important and opposite roles in the regulation of cardiovascular and metabolic homeostasis. Leptin/adiponectin ratio (LAR) has recently been proposed as a new atherogenic index4.
Furthermore, human adipocytes produce an as yet unidentified mineralocorticoid-releasing factor that stimulates adrenal aldosterone production5 and this hormone in turn promotes the release of pro-inflammatory adipocytokines that cause systemic inflammation, oxidative stress and insulin resistance6. In fact, obesity is associated with an increase in aldosterone production and high levels of this hormone promote the development of insulin resistance7.
Plasma aldosterone levels have emerged as an indicator of adverse prognosis in several cardiac and noncardiac conditions, including heart failure8 and acute myocardial infarction9. There are few studies measuring aldosterone levels in haemodialysis (HD) patients.
Drechsler and colleagues determined aldosterone levels in 1,255 diabetic patients on dialysis and showed that high aldosterone levels were associated with an increased risk of all-cause mortality as well as sudden cardiac death10. Hung and colleagues in a prospective cohort study in 328 HD patients showed that SA levels were inversely associated with adverse outcomes; volume overload underlies this paradox. In the absence of volume overload, SA was an independent risk factor for all-cause mortality and CV events in this population11.
We hypothesised that adipose tissue could promote the release of aldosterone by mechanisms independent of the RAAS, and that aldosterone might contribute to the pathogenesis of CV disease in dialysis patients by altering the pattern of secretion of fatty tissue adipocytokines, and consequently promoting insulin resistance.
The aim of this study was to measure plasma aldosterone levels in patients on HD and the possible association with excess body fat (bioimpedance) and/or serum adipocytokine levels.
PATIENTS AND METHODS
This is a cross-sectional study performed in April 2015. Subjects were recruited among chronic HD patients at the Hospital General (Segovia, Spain) and its affiliated centre: Los Olmos (FRIAT). Selection criteria included: age >18 years; patient on HD for at least six months; not to have: a) diabetes mellitus b) any intercurrent acute illnesses in the last three months; c) data from dialysis treatment; d) inability to perform bioimpedance owing to metallic prostheses or amputations; e) treatment with drugs that interfere with RAAS (including angiotensin receptor blockers (ARB), angiotensin converting enzyme (ACE) inhibitor and aldosterone receptor blockers).
All measurements were taken fasting and with the patient prone, prior to the midweek HD session. The following studies were performed in every patient:
1. Multifrequency whole body bioimpedance assessment using the Body Composition Monitor (Fresenius Medical Care, Bad Homburg, Germany). We measured lean tissue index (LTI); fat tissue index (FTI); relative fat mass (kg/m²); total water (TW); extracellular water (ECW), intracellular water (ICW). We estimated relative overhydration (rel OH) calculating the difference between initial body weight (Kg) and dry body weight measured with BCM.
2. A comprehensive biochemical and haematological study that included serum levels of glucose; albumin; cholesterol; transferrin; haematocrit; creatinine; sodium and potassium; pH; bicarbonate; and ultrasensitive C reactive-protein (CRPs), all measured by routine methodology in our laboratory.
3. Dialysis dose was estimated using Kt/V Daugirdas II formula; and protein ingestion using the protein catabolic rate normalised to actual body weight (nPCR).
4. The volume of diuresis and the residual renal function were calculated by collecting 24-hour urine the day prior to the midweek HD session.
5. Hormonal study. Blood samples were immediately centrifuged, separated and frozen at –80ºC. All hormones were determined simultaneously in a centralised laboratory using specific commercial methods. Serum aldosterone (SA) was determined in plasma EDTA by chemiluminescent competitive assay (LIAISON®, Dia Sorin); intra and inter assay coefficient of variation were 3.5% and 9.5% for an average value of 6.8 ng/dl and 1.8% and 5.6% for an average value of 28.8 ng/dl, respectively; SA normal range for our laboratory is 1.17-23.6 ng/dl. Plasma renin activity (PRA) was determined in plasma EDTA, by chemiluminescent assay (LIAISON®, Dia Sorin); intra and inter assay coefficient of variation were 2.1%-2.38% for an average value of 2.25 ng/ml/h and 6.8%-7.3% for an average value of 8.25 ng/ml/h, respectively; PRA normal range for our laboratory is 0.23-3.32 ng/ml/h. Leptin was determined by ELISA (enzyme immunoassay) in the DSX Palex (DRG) ng/ml; the intra and inter assay coefficient of variation were 5.9%-6.9 and 8.6-11.5%, respectively; its normal range is 7.36±3.73 ng/ml in females and 3.84±1.79 ng/ml in males. Adiponectin was determined by ELISA (enzyme immunoassay) in DSX Palex (Millipore) ng/ml; variation coefficient intra and inter assay were 2.4-8.4 and 1.9-7.4%, respectively.
6. Comorbidity was assessed using the Charlson Index, as modified by Beddhu12. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS), Version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Normal data distribution was explored by the Kolmogorov-Smirnov test. Data are given as means ± SD for continuous variables with normal distribution or as percentages for categorical variables. SA levels and ARP were normally distributed. The Chi-squared test and Fishers exact test were used to compare proportions. Students t or Mann-Whitney U test were used to compare means. Pearson`s test was used to establish linear correlation coefficients; if any variable was ordinal or didnt have a normal distribution we used Spearman´s rho. To analyse the factors related to SA levels we used a stepwise multiple regression analysis, including those variables that were significant in the univariate analysis as well as confounding factors (age, sex, comorbidity index, and history of cardiac disease). A p value of less than 0.05 was considered significant.
A written informed consent was obtained from all study patients. The study is in accordance with the declaration of Helsinki about Ethical Principles for Medical Research Involving Human Subjects.
RESULTS
Socio-demographic, clinical and anthropometric characteristics of the 28 patients included in our study are shown in table 1. All HD patients were dialysed with synthetic membranes and high flow dialysis. 18 patients were anuric (residual volume of diuresis <100ml/24 hours).
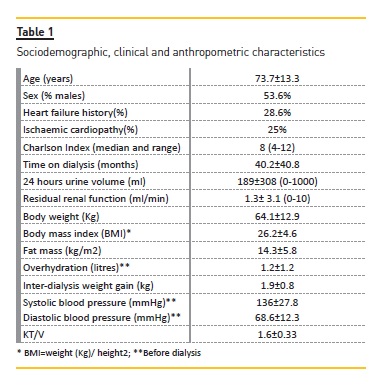
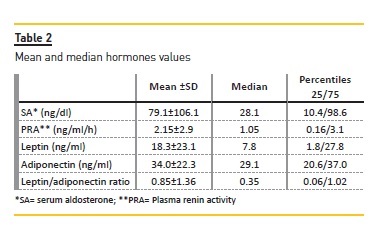
Table 3 shows the characteristics of patients with SA levels above or below the median. Patients with SA above the median had significantly higher RPA and LAR and lower levels of adiponectin. Patients with higher SA had higher body weight, body mass index, relative fat mass and leptin levels although the differences were not statistically significant.
There were no statistically significant differences in SA levels between anuric and non-anuric, male and female and presence and absence of myocardiopathy.
There were no statistically significant correlations of SA levels or PRA with urine volume, RRF, systolic or diastolic blood pressure, relative overhydration, dose of HD or time on dialysis, age or comorbidity.
SA levels were positively correlated with PRA (r= 0.70; p< 0.0001); body fat mass in kg/m2 (r=0.40; p=0.03); leptin (r = 0.45; p = 0.01); leptin/adiponectin ratio (r=0.52 p=0.005) and negatively with serum adiponectin levels (r= – 0.37; p=0.05).
To analyse the factors related to SA levels we used a stepwise multivariate regression analysis, including variables that were significant in the univariate analysis as well as confounding factors (age, sex, comorbidity index and history of cardiac disease). The best model to explain SA levels included PRA and LAR (r=0.76; r2=0.57).
DISCUSSION
Our study shows that SA levels are elevated in a high percentage of patients on a chronic HD programme and that these levels are correlated with PRA but also with body fat mass and adipocytokines. These findings lead us to questions, such as:
1. Is aldosterone activity increased in HD patients?
2. What is the role of adipose tissue and adipocytokines in the development of hyperaldosteronism?
3. Is aldosterone involved in the cardiovascular and metabolic adverse effects attributed to fat tissue?
Activity of the renin-angiotensin-aldosterone system in HD patients
An important finding of this study is that almost 80% of our patients had normal or elevated PRA, although most of them were anuric or had very low RRF. Our results confirm that PRA is the main factor related to the levels of AS which were above the normal range in 53.6% of patients.
In our study, no patient was being treated with ACE inhibitors or ARBs. In a previous study including 102 patients in dialysis, 28 patients treated with those drugs showed significantly lower levels of AS (data not shown). These data confirm that at least part of the hyperaldosteronism of dialysis patients is due to the activation of the RAAS and could help explain why the pharmacological blockade with ACE inhibitors / ARBs has a beneficial effect in preventing CV risk in many HD patients as shown by some authors13-16.
The role of adipose tissue and adipocytokines in the development of hyperaldosteronism
Our results show that aldosterone levels correlated positively with body fat mass (kg/m2). Several studies have shown that adipose tissue, through unidentified factors, can stimulate the secretion of aldosterone from the adrenal gland17,18. In an interesting piece of research Kidambi and colleagues19 compared the levels of SA and PRA in patients with and without metabolic syndrome (MS). Their results show no difference in PRA between both groups but patients with MS had significantly higher levels of SA, suggesting that in those patients a different mechanism to RAAS is responsible for the secretion of SA. The authors propose that adipose tissue is responsible for the secretion of aldosterone, independently of RAAS, and furthermore that aldosterone binding to MCR cause insulin resistance.Is aldosterone involved in the cardiovascular and metabolic adverse effects attributed to fat tissue?
In our study we found a positive correlation between aldosterone levels and leptin and LAR, and a negative one with adiponectin. In addition, in the stepwise multiple regression analysis, LAR independently correlated with the levels of aldosterone. Adipose tissue is not only a store of excess energy but also a complex, essential and highly active metabolic and endocrine organ.
The role of body fat mass in the development of uraemic insulin resistance can be attributed to adipocytederived hormones such as leptin and adiponectin, with opposing effects in regulating metabolism. Leptin, a protein that is secreted exclusively by adipocytes, plays an important role in insulin resistance3,20 and adiponectin has anti-inflammatory, insulin-sensitizing, anti-atherogenic properties and as such a protective effect on CV disease21. An inverse correlation between adiponectin and insulin resistance, assessed by the HOMA-R index, has been reported in non-uraemic22 and uraemic subjects23.
In dialysis patients the levels of leptin and adiponectin are elevated, and the reduction in leptin renal clearance seems to be the main cause of this finding24. The prognostic significance of these adipocytokines levels in dialysis patients is controversial. Most authors have found a protective effect of adiponectin on CV mortality in dialysis patients25 although some have found the opposite effect26. Recently Hung et al.27 have shown that leptin/adiponectin ratio (LAR) has a good correlation with glucose-disposal rate measured by hyperinsulinemic-euglycemic glucose clamp in chronic HD patients, and recently LAR has been proposed as a new atherogenic index since it has been associated with cardiovascular events in PD4.
In this study we found an independent association between aldosterone levels and LAR index. Given that this LAR index has a good correlation with insulin resistance in patients with HD, we infer that aldosterone could play a pathogenic role in the development of insulin resistance but our data do not allow us to conclude a causal relationship. The association between hyperaldosteronism and insulin resistance was suggested 50 years ago when it was reported that patients with primary aldosteronism had an impaired glucose tolerance28 and that tumour resection or pharmacological treatment reduced insulin levels (29,30). The mechanisms by which aldosterone can lead to insulin resistance are not fully defined. There are MCR in skeletal muscle, pancreatic beta cells, liver and adipose tissue and their activation might lead to insulin resistance by decreasing insulin secretion, increased hepatic gluconeogenesis and release of adipocytokines. Emerging evidence supports a paradigm shift in our understanding of the RAAS and in aldosterones ability to promote insulin resistance31. Research in animal models, with activated RAAS and insulin resistance, showed that blocking of MCR improved sensitivity to insulin and the uptake of glucose by skeletal muscle32. A recent study shows that the use of ACE inhibitors or ARBs on dialysis improves insulin sensitivit33.
Our study is limited by its cross-sectional design and sample size, which does not allow us to make comparisons between obese and non-obese patients. It would be necessary to measure HOMA-IR or hyperinsulinemiceuglycemic clamp to establish the causal relationship between aldosterone and insulin resistance. A prospective study with higher numbers of patients is needed to see whether blocking MCR decreases insulin resistance in patients with excess body fat or elevated LAR.
CONCLUSIONS
SA levels were elevated in a high percentage of HD patients. In this study, almost 80% of the HD patients had normal or elevated PRA, although most of them were anuric or had a very low RRF. SA levels were correlated with PRA.Our results confirm that PRA is the main factor related to SA levels of, although RAAS activation is paradoxical since it does not correlate with pre-dialysis hydration. SA levels were also associated to body mass fat and levels of adipocytokines. In the stepwise multiple regression analysis LAR was independently correlated with the levels of SA. In chronic HD patients LAR has a good correlation with insulin resistance parameters, so our results suggest but cannot prove a cause-effect relationship between SA and insulin resistance.
References
1. Tonelli M, Wiebe N, Culleton B et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006;17:2034–2047. [ Links ]
2. Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int 1999;55:1560–1567. [ Links ]
3. Hung SC, Tarng DC. Adiposity and insulin resistance in nondiabetic hemodialysis patients:effects of high energy supplementation. Am J Clin Nutr 2009;90:64–69. [ Links ]
4. Bernardo AP, Fonseca I, Oliveira JC, Santos O, Carvalho MJ, Cabrita A, Rodrigues A. Adipokines in peritoneal dialysis: relevant clinical impact according to body composition. Ther Apher Dial 2015;19:144–153. [ Links ]
5. Krug AW, Ehrhart-Bornstein M. Adrenocortical dysfunction in obesity and the metabolic syndrome. Horm Metab Res 2008;40:515–517. [ Links ]
6. Ehrhart-Bornstein M, Arakelyan K, Krug AW, Scherbaum WA, Bornstein SR. Fat cells may be the obesity-hypertension link: human adipogenic factors stimulate aldosterone secretion from adrenocortical cells. Endocr Res. 2004;30:865–870. [ Links ]
7. Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med 2009;150(11):776–783. [ Links ]
8. Guder G, Bauersachs J, Frantz S, et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 2007;115:1754–1761. [ Links ]
9. Palmer BR, Pilbrow AP, Frampton CM, et al. Plasma aldosterone levels during hospitalization are predictive of survival post-myocardial infarction. Eur Heart J 2008;29:2489–2496. [ Links ]
10. Drechsler C, Ritz E, Tomaschitz A, et al. Aldosterone and cortisol affect the risk of sudden cardiac death in haemodialysis patients. Eur Heart J 2012;34:578–587. [ Links ]
11. Hung SC, Lin YP, Huang HL, Pu HF, Tarng DC. Aldosterone and mortality in hemodialysis patients: role of volume overload. PLoS One 8 2013;e57511. [ Links ]
12. Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcome and cost in dialysis patients. Am J Med 2000;108(8):609-613. [ Links ]
13. Takahashi A, Takase H, Toriyama T, Sugiura T, Kurita Y, Ueda R, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis–a randomized study. Nephrol DialTransplant 2006;21(9):2507–2512. [ Links ]
14. Suzuki H, Kanno Y, Sugahara S, Ikeda N, Shoda J, Takenaka T, et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis 2008;52(3):501–506. [ Links ]
15. Yang LY, Ge X, Wang YL, Ma KL, Liu H, Zhang XL, et al. Angiotensin receptor blockers reduce left ventricular hypertrophy in dialysis patients: a meta-analysis. Am J Med Sci 2013;345(1):1–9. [ Links ]
16. Tai D, Lim T, James M, Manns B, Tonelli M, Hemmelgarn B, et al. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol 2010;5(4):623–630. [ Links ]
17. Lamounier-Zepter V, Ehrhart-Bornstein M. Fat tissue metabolism and adrenal steroid secretion. Curr Hypertens Rep 2006;8:30–34. [ Links ]
18. Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension 2004;43:358–363. [ Links ]
19. Kidambi S, Kotchen J, Grim C, Raff H, Mao J, Singh R,Kotchen T. Association of adrenal steroids with hypertensionand the metabolic syndrome in blacks. Hypertension 2007;49:704–711. [ Links ]
20. Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004;15:2792–2800. [ Links ]
21. Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care 2003;26(8):2442-2450. [ Links ]
22. Lu JY, Huang KC, Chang LC, Huang YS, Chi YC, Su TC, et al. Adiponectin: a biomarker of obesityinduced insulin resistance in adipose tissue and beyond. J Biomed Sci 2008;15(5):565–576. [ Links ]
23. Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Buemi M, Nicocia G, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol 2002;13,134–141. [ Links ]
24. Díez JJ, Iglesias P, Fernández-Reyes MJ, Aguilera A, Bajo MA. Alvarez-Fidalgo P, Codoceo R and Selgas R. Serum concentrations of leptin, adiponectin and resistin, and their relationship with cardiovascular disease in patients with end-stage renal disease. Clinical Endocrinology 2005;62:242–249. [ Links ]
25. Díez JJ, Estrada P, Bajo MA, Fernández-Reyes MJ, Grande C, del Peso G, Heras M, Molina A, Iglesias P, Sánchez-Villanueva R, Selgas R. High stable serum adiponectin levels are associated with a better outcome in prevalent dialysis patients. Am J Nephrol 2009;30(3): 244-252. [ Links ]
26. Rhee CM, Nguyen DV, Moradi H, Brunelli SM, Dukkipati R, Jing J, Nakata T, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis 2015;66(2):313-321. [ Links ]
27. Hung AM, Sundell MB, Egbert P, Siew ED, Shintani A, Ellis CD, Bian A, Ikizler TA. A comparison of novel and commonly-used indices of insulin sensitivity in African American chronic hemodialysis patients. Clin J Am Soc Nephrol 2011;6:767–774. [ Links ]
28. Conn JW. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med 1965;273:1135–1143. [ Links ]
29. Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens 2007;25:177–186. [ Links ]
30. Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab 2006;91:3457–3463. [ Links ]
31. Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Reninangiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 2007;293:H2009–2023. [ Links ]
32. Lastra G, Whaley-Connell A, Manrique C, Habibi J, Gutweiler AA, Appesh L, et al. Lowdose spironolactone reduces reactive oxygen species generation and improves insulinstimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab 2008;295(1):E110-116. [ Links ]
33. Satirapoj B, Leelasiri K, Supasyndh O, Choovichian P. Short-term administration of an angiotensin ii receptor blocker in patients with long-term hemodialysis patients improves insulin resistance. J Med Assoc Thai 2014;97(6):574-581. [ Links ]
Mª José Fernández-Reyes
Nephrology Department. Complejo Asistencial de Segovia.
C/ Luis Erick Claveria Neurólogo.40100-Segovia.Spain
Fax +34 91 419169. Tel. +34 91419170
E‑mail: mfernandez@hgse.sacyl.es
Disclosure of potential conflicts of interest: This study was partially performed with the help of a research grant from SACYL (Biomedical Research Project) GRS 990/A/14. The results presented in this paper have not been published previously in whole or part.
Acknowledgements
We thank Dr. F. Alvarez–Ude for helping in the writing of this manuscript.
Received for publication: Mar 2, 2016
Accepted in revised form: May 20, 2016














