Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.4 Lisboa dez. 2017
ORIGINAL ARTICLE
Are we building too many arteriovenous fistulas? A single-center experience
Carla Leal Moreira1, Vanda Teixeira1,2, Lígia Bessa1,2, José Queirós1, Fernanda Silva1, António Cabrita1
1 Nephrology Department, Centro Hospitalar Universitário do Porto, Hospital Geral de Santo António
2 Nephrology Department, Hospital Militar de Luanda
ABSTRACT
Introduction: Arteriovenous fistula has been associated with improved morbimortality in hemodialysis patients.
This has resulted in the fistula First, catheter last initiative. Nonetheless, the survival benefit of arteriovenous fistula has been questioned.
Methods: We conducted a retrospective observational study of all patients with non-end stage renal disease referred for first vascular access building between January 2014 and December 2015 in our hospital center. Our main goal was to evaluate the clinical impact and burden of building fistula in predialysis patients.
Results: During this period, of 178 first arteriovenous accesses placed, 87 patients remained in predialysis and 91 patients started a chronic hemodialysis program. Median follow-up time by a nephrologist was 3.9 (2.5, 9.7) years. The mean age was 65.8±14.7 years, with 50.6% (n=90) of male patients. A higher rate of thrombosis in the predialysis group (26% vs 13%, p=0.037) was observed, but vascular access survival did not differ significantly (55% vs 67%, p=0.12). Mean vascular access placing was higher in the predialysis group (1.4±0.7 vs 1.2±0.4, p=0.006) and less interventions were requested (0.2±0.5 vs 0.3±0.6, p=0.10). Median time from vascular access placement to hemodialysis start was 22 (13, 41) months. At hemodialysis initiation, 10 (10.9%) patients used a central venous catheter; 80 (87.9%) patients an arteriovenous fistula, and one patient a graft. A total of 227 vascular accesses were built; 121 (53.3%) in predialysis vs 106 (46.7%) in incident hemodialysis patients. In a multivariate model, the presence of a functional arteriovenous fistula at hemodialysis start was only associated with a trend to survival benefit (HR 0.38, 95% CI 0.14-1.00, p=0.05).
Conclusions: Our results stress the need for an individual approach and for future tools to assess the risk of death and progression to end-stage renal disease, therefore helping reduce the number of unutilized vascular accesses and rising cost of interventions.
Keywords: Arteriovenous Fistula; Central Venous Catheter; Morbidity; Survival
INTRODUCTION
The general recommendation for vascular access (VA) placement in hemodialysis patients has been fistula first.1-4
Historically, a survival benefit of arteriovenous fistula (AVF) over arteriovenous graft (AVG) and tunneled dialysis catheter (TDC) has been suggested.5-8 This has been the main argument for guidelines such as the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) and the Society for Vascular Surgery prioritizing AVF as the ideal long-term vascular access; AVG as the following preferred vascular access choice, and TDC the last option.9,10 Furthermore, these recommendations are supported by available data suggesting AVF has superior patency, fewer complications, less need of re-interventions, and ultimately improved patient survival.2,11
Timely referral and placing of arteriovenous VA is one of the best preventive measures of catheter use at hemodialysis initiation, but defining the ideal timing of arteriovenous (AV) access placement, namely of AVF, remains challenging and not consensual10,12,13. Questions start to be raised when non-functionality rates of about 18% have been reported among the overall incident hemodialysis patients in Canada and the US populations, while a French study reported a rate of 9% nonfunctional AVF14-17. A nonfunctional AVF is associated with increased access-related complications and procedures18. Moreover, prior literature that describes the benefit of AVF over AVG and TDC is based on the access first used as opposed to the access first placed19.
Furthermore, the competing risk of death must be considered, especially in the elderly, as it results in a considerable number of patients with matured AVF who die before even starting hemodialysis18.
We aimed to retrospectively review the impact of vascular access placement in a cohort of predialysis patients in both vascular access-related complications and survival, and patient survival.
METHODS
We retrospectively reviewed all patients in predialysis who were referred to our specialized consulting at Centro Hospitalar Universitário do Porto, between January 2014 and December 2015, for preoperative physical examination, blood vessel assessment by doppler ultrasound (DU), and vascular access planning. Only firsttime referral for AV accesses were included in this review. Patients who had undergone prior VA surgery or who were re-starting hemodialysis (after kidney transplant, for instance) were excluded from the analysis.
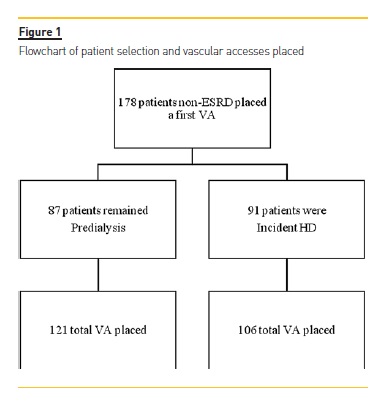
The presence of a temporary or cuffed dialysis catheter was not an exclusion criterion. A total of 178 patients were included (Figure 1).
DU measurements were taken by a single nephrologist skilled in DU. The type of VA proposed by the consulting nephrologist was dependent on patient past medical history, physical examination, and DU findings20.
The final choice of VA selected could be altered due to findings during the surgical proceedings. All patients were informed about the planned procedure and gave informed consent.
Hospital registries were reviewed for patient demographics, pertinent medical history, physical evaluation findings, preoperative imaging studies, and findings from the postoperative follow-up. All postoperative interventions were reviewed for indication, procedure type, and outcome. Patient was considered to carry a functional AVF when it could deliver adequate blood flow and was ready to cannulate at hemodialysis (HD) start.21 The end of VA follow-up was the beginning of HD for those enrolled in a chronic hemodialysis program.
VA follow-up in patients who remained predialysis ended at the time of death or the first of November of 2016. All patients follow-up ended on the first of November of 2016.
Our primary endpoint was to assess the timing, complications, and survival of first VA placed in patients with non-end-stage renal disease (ESRD) referred to VA creation. Secondary endpoints were progression to ESRD and patient survival.
Chi-square test was used to compare categorical variables. Kolmogorov-Smirnovs test was performed to assess deviation from normal distribution. T test was used for normally distributed continuous variables. Mann-Whitney was applied to skewed variables. Logrank test was used to assess VA and patient cumulative survival, and respective curves were derived by Kaplan-Meier method. Multivariate analysis of patient and VA survival was performed using Cox regression; only clinically relevant or statistically significant variables were included in the model. All tests of significance were two-sided and differences were considered significant when p≤0.05. Data are reported as percentages, mean ± standard deviation (SD) or median and interquartile range (IQR) and 95% confidence interval (CI) as appropriate. All statistical analyses were performed using Stata/IC 14.0®.
RESULTS
Between January 1st, 2014 and December 31th, 2015, 178 adult patients with non-ESRD were evaluated for first VA creation. The mean age was 65.8±14.7 years, with 62.9% being older than 65 years-old, and 29.2% older than 75 years-old. Male patients represented 50.6% (n=90). Patients with history of diabetes represented 45.5% (n=81), ischemic heart disease (IHD) 25.3% (n=45), peripheral artery disease 19.1% (n=34), and cerebrovascular disease 31.5% (n=56). Median follow-up time by a nephrologist was 3.9 (IQR 2.5, 9.7) years and mean estimated glomerular filtration rate at referral was 16.8±5.2 ml/min/1.73m2. Baseline characteristics of patients who progressed and did not progress to ESRD are presented in table I.
It was possible to construct a VA in all patients; 177 AVFs and one AVG. Median time from VA referral and VA placement was 22 days (IQR 13, 37). In 19 (10.7%) patients, VA placed differed from the one suggested by the Nephrologist. There were 86 (48.3%) radiocephalic AVF, 65 (36.5%) brachio-cephalic AVF, and 26 (14.6%) brachio-basilic AVF. Median VA follow-up was 24.4 (IQR 8.1, 46.9) months. One hundred and nine VA (61.2%) were patent at the end of follow-up; 52 (47.7%) radio-cephalic AVF, 42 (38.5%) brachio-cephalic AVF,14 (12.8%) brachio-basilic, and one AVG. By the end of the follow-up only 91 (51.1%) patients started hemodialysis and 21 (11.8%) patients died before starting hemodialysis. Median time from VA placement and HD initiation was 22 (IQR 13, 41) months, 14 (7.9%) patients had the VA placed <6 months before HD start, 14 (7.9%) patients between 6-12 months before HD start, and 150 (84.3%) more than one-year earlier. The first complication experienced was primary failure in twenty-five (14.0%) patients, 20 (11.2%) AVF thrombosed, with 40 (22.5%) AVF being abandoned. At the end of the study 26% (n=23) vs 13% (n=12) of first AVF thrombosed in predialysis and HD group, respectively (p=0.037).
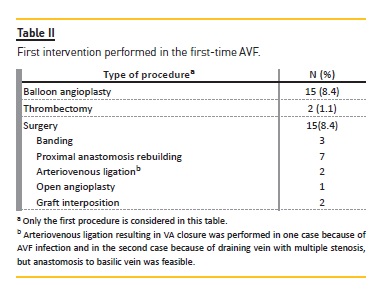
Eighty-four patients diagnosed with a potentially rectifiable VA complication underwent a total of 48 interventions during the follow-up period (Table II); 29(16.3%) patients experienced one intervention, 5 (2.8%) patients were submitted to 2 interventions, and 3 (1.7%) patients underwent 3 procedures. Main vascular access characteristics are described in table III. Of those starting hemodialysis, 10 (10.9%) patients started with TDC, 80 (87.9%) with AVF, and one with AVG. A total of 227 VA was built, of which 121 (53.3%) were in the predialysis group and 106 (46.7%) in incident HD patients, with 35 (19.6%) patients submitted to 2 VA placement, 4 (2.2%) patients submitted to 3 VA placement and 2 patients submitted to 4 VA placement.
At the end of VA follow-up, 48 (55%) patients in predialysis and 61 (67%) patients in the group who started HD (p=0.12) had their first VA access patent. In a multivariate model including age, sex, diabetes, peripheral artery disease, cerebrovascular disease, ischemic heart disease (IHD), DU diameter of nurturing artery and outflow vein, and number of VA-related interventions, only IHD (HR 1.97, 95% CI 1.06-3.66, p=0.03) and cerebrovascular disease (HR 1.99, 95% CI 1.08-3.67, p=0.03) were significantly associated with worse VA survival.
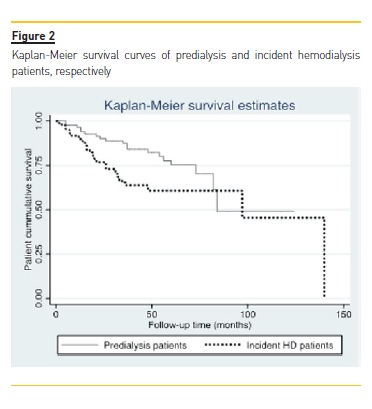
Patient cumulative survival in the group who remained predialytic was 76% (n=66) and 70% (n=64) for those starting chronic HD (p=0.50). The presence of a functioning AVF at HD start did not correlate with a statistically significant improved survival (HR 0.47, 95% CI 0.17-1.24, p=0.129). But in a multivariate analysis including gender, age, IHD, peripheral arterial disease, cerebrovascular disease, diabetes, and presence of functional AVF, age was associated with increased mortality (HR 1.06, 95% CI 1.01-1.11, p=0.008), while functional AVF at HD start showed a trend towards improved survival (HR 0.38, 95% CI 0.14-1.00, p=0.05). When all patients were included in the model (HD and predialysis), only age was significantly associated with worse prognosis (HR 1.07, 95% CI 1.03-1.11, p<0.0001). Patient cumulative survival curves can be observed in Figure 2.
DISCUSSION
Optimizing vascular access outcomes continues to be an uppermost priority in the management of non-ESRD and hemodialysis patients. The initial KDOQI guidelines published in 19971 and updated in 20012 and 20069 as well as the Fistula First Initiative in 20033 have consistently recommended preferential placement of AVFs over AVGs or TDCs.
Several rationales for preferring AVFs over AVGs have been proposed: (1) AVFs have better longevity, (2) require fewer interventions to maintain long-term patency, (3) lower access-related costs, and (4) HD patients with an AVF have a lower mortality4,11,22.
The Fistula First Initiative has been questioned, particularly in the elderly. A recent study revealed that patients older than 80 years-old had similar survival outcomes whether an AVF or AVG was placed first; TDC had clearly the inferior outcome, and only50.7% of the patients who had an AVF as their first access placed actually used an AVF at the time of dialysis initiation15. Another recent observational study documented that older patients (≥67yo) who initiate HD with a central venous catheter (CVC), who had an AVF placed rather than an AVG, within 6 months of dialysis initiation, were associated with better overall survival, despite longer CVC dependence.
Moreover, patients with AVF placement had lower rates of hospitalization due to all-cause infection and septicemia/bacteremia.23
In our study two thirds of the patients were older than 65 years-old, one third of them older than 75 years-old, while showing an impressive rate of AVF use at HD initiation (87.9%). This happened at the expense of 227 arteriovenous (AV) accesses placed in 178 patients, with only 91 patients engaging HD and 21 (11.8%) deaths before even starting HD, in a 4-year period of follow-up.
Additionally, discrepancies remain in guidelines for optimal timing of vascular access placement: K/DOQI9 guidelines suggest at least 6 months before HD start; the European Best Practice Guidelines12 recommend AVF creation at least 2-3 months before hemodialysis start, whereas the Society for Vascular Surgery10 defines a glomerular filtration rate of 20 to 25 ml/min/1.73m2 as the criterion for fistula referral.
The timely creation of an AV access in patients with non-ESRD requires consideration of factors, such as patient preferences, life expectancy, likelihood of needing and timing of hemodialysis start, AV access eligibility, and risk of complications. A Canadian populationbased study showed, when late-referral patients were excluded, that 39% of the remaining cohort had a predialysis AV access creation and 27% used an AV access at hemodialysis, with median time between AV access creation and hemodialysis start of 184 days.16 A French study based in the REIN registry documented an overall 18% patients with nonfunctional AV access at hemodialysis initiation; in those with a planned dialysis start, the interval between AV access creation and hemodialysis initiation was <1 month for 14% of patients, 1–3 months for 27%, 3–12 months for 38%, and >12 months for 21%.17
Our results revealed a median time from VA placement and HD initiation of 22 months, 84.3% built a VA more than one-year before HD initiation, while 7.9% patients had the VA placed between 6-12 months, and 7.9% <6 months before HD start, respectively. This finding is possibly related to challenges in the anticipation of HD onset because of nonlinear chronic kidney disease progression, reversible acute kidney injury and the ultimate concern for a functional AVF at the time of HD initiation. A strategy of closer follow-up of patients eligible for an AVF may facilitate determination of the ideal timing for AV access creation. Optimal timing should also involve the avoidance of AV access creation in patients who will not begin dialysis.
A decision analysis assessing the ideal timing for AV access referral through Monte Carlo simulation found that earlier referral for older patients had virtually no impact on rates of successful AV access use at hemodialysis initiation, but increased the frequency of unnecessary AV access creation.24 This outcome was due mainly to mortality before hemodialysis initiation, which suggests that early VA placement in patients with a high comorbidity burden may often be pointless. Furthermore, a population-based study conducted in Canada showed that patients with a higher Deyo-Charlson Comorbidity Index were more likely to start hemodialysis after AVF creation, when age, sex, and the competing risk of death were considered.25
We registered 38.8% (n=69) non-functional AVF at the end of the study. In the multivariate analysis only IHD and cerebrovascular disease were associated with worse VA survival. The reason for the rate of failure seen in our cohort might be explained by an exhaustive attempt to create autologous fistulas. It has been proposed that, in the elderly, an AVG may be a preferable choice of second VA in a specific group of patients, such as females, blacks, and those with peripheral vascular disease.26 In addition to a clinical-tree decision, DU assessment may help select the best VA option for each individual patient.
Our study has some limitations. First, due to its observational nature we are only able to infer associations rather than causality. Second, our study is probably underpowered to detect a survival benefit, yet we did find a marginal survival improvement from functional AVF at HD initiation, with age being the most important mortality predictor in our model.
Brown et al. and Quinn et al. described that among patients initiating HD with a CVC, those who underwent AVF placement before HD initiation had superior survival to those without predialysis access surgery, despite having a very similar profile of comorbidities.27,28 This was even if the AVF failed to mature and was never used for HD, and thus with no correlation with a shorter CVC dependence period. This finding suggests that the decision to place an AVF in a patient itself reflects a healthier patient in ways that are not captured by compiling a list of known comorbidities. Moreover, an observational study suggested that the association between access type and mortality was nearly identical in models excluding and including access complications (HR 2.00; 95% CI 1.55-2.58 vs HR 2.01 95% CI 1.56-2.59 for CVC versus AVF, respectively).29
Regarding costs, a recent study suggests that even though AVF have been considered the most cost-effective option, this benefit decreases with rising age and lower life expectancy.30
In conclusion, a high AVF placement rate was possible in the K/DOQI era. Nevertheless, the improving number of functional AVF can be at the expense of an exceeding number of futile VA created, increased rate of failing AVFs and rising burden of interventions. To date no randomized controlled trial has been designed to access the real benefit from predialysis AVF placement.
Our results highlight the aging of our population and the need for an individual approach and for future tools which can help predict the risk of death and progression to ESRD.
References
1. National Kidney Foundation-Dialysis Outcomes Quality Initiative. NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis 1997;30(3):S150–S191. [ Links ]
2. NKF-K/DOQI: III. NKF-K/DOQI clinical practice guidelines for vascular access: update 2000. Am J Kidney Dis 2001;37(1):S137–S181. [ Links ]
3. Lok CE. Fistula first initiative: advantages and pitfalls. Clin J Am Soc Nephrol 2007;2:1043–1053. [ Links ]
4. Allon M. Current management of vascular access. Clin J Am Soc Nephrol 2007;2:786–800. [ Links ]
5. Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 2001;60:1443–1451. [ Links ]
6. Woods JD, Port FK. The impact of vascular access for haemodialysis on patient morbidity and mortality. Nephrol Dial Transplant 1997;12:657–659. [ Links ]
7. Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 2004;15:477–486. [ Links ]
8. Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 2003;42:1013–1019. [ Links ]
9. National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 updates: hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis 2006;48(1):S1–S322. [ Links ]
10. Sidawy AN, Spergel LM, Besarab A, Allon M, Jennings WC, Padberg FT Jr, et al. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg 2008;48(5 Suppl):2S–25S. [ Links ]
11. Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 2002;62:1109– 1124. [ Links ]
12. Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, et al. EBPG on Vascular Access. Nephrol Dial Transplant 2007;22(2):ii88–117. [ Links ]
13. 2006 Updates Clinical Practice Guidelines and Recommendations [Internet]. Available at: https://www.kidney.org/sites/default/files/docs/12-50-0210_jag_dcp_guidelinesva_oct06_sectionc_ofc.pdf. [cited Jan 5 2016]. [ Links ]
14. Grubbs V, Wasse H, Vittinghoff E, Grimes BA, Johansen KL. Health status as a potential mediator of the association between hemodialysis vascular access and mortality. Nephrol Dial Transplant 2014;29(4):892–898. [ Links ]
15. Malas MB, Canner JK, Hicks CW, Arhuidese IJ, Zarkowsky DS, Qazi U, et al. Trends in incident hemodialysis access and mortality. JAMA Surg 2015;150(5):441–448. [ Links ]
16. Al-Jaishi AA, Lok CE, Garg AX, Zhang JC, Moist LM. Vascular access creation before hemodialysis initiation and use: a population-based cohort study. Clin J Am Soc Nephrol 2015;10(3):418–427. [ Links ]
17. Alencar de Pinho N, Coscas R, Metzger M, Labeeuw M, Ayav C, Jacquelinet C, Massy ZA, Stengel B; French REIN registry. Vascular access conversion and patient outcome after hemodialysis initiation with nonfunctional arteriovenous access: a prospective registrybased study. BMC Nephrol. 2017 Feb 22;18(1):74. [ Links ].
18. DeSilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, et al. Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol 2013;24(8):1297–1304. [ Links ]
19. Yuo TH, Chaer RA, Dillavou ED, Leers SA, Makaroun MS. Patients started on hemodialysis with tunneled dialysis catheter have similar survival after arteriovenous fistula and arteriovenous graft creation. J Vasc Surg 2015;62(6):1590-1597.e2. [ Links ]
20. Barreto P, Almeida P, de Matos N, Queirós JA, Pinheiro J, Silva F, et al. Preoperative vessel mapping in chronic kidney disease patients – a center experience. J Vasc Access 2016;17(4):320–327. [ Links ]
21. Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M, Jr, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 2002;35:603–610. [ Links ]
22. Lok CE, Foley R. Vascular access morbidity and mortality: trends of the last decade. Clin J Am Soc Nephrol 2013;8:1213–1219. [ Links ]
23. Lee T, Thamer M, Zhang Q, Zhang Y, Allon M. Vascular access type and clinical outcomes among elderly patients on hemodialysis. Clin J Am Soc Nephrol 2017;10.pii: CJN.01410217.
24. Shechter SM, Skandari MR, Zalunardo N. Timing of arteriovenous fistula creation in patients with CKD: a decision analysis. Am J Kidney Dis 2014 Jan;63(1):95-103. [ Links ]
25. Oliver MJ, Quinn RR, Garg AX, Kim SJ, Wald R, Paterson JM. Likelihood of starting dialysis after incident fistula creation. Clin J Am Soc Nephrol 2012;7(3):466–471. [ Links ]
26. Hod T, Goldfarb-Rumyantzev AS, Patibandla BK, Narra A, Brown RS. Second vascular access after failure of the first fistula in the elderly. Clin Nephrol 2016;86:253–261. [ Links ]
27. Brown RS, Patibandla BK, Goldfarb-Rumyantzev AS. The survival benefit of Fistula First, Catheter Last in hemodialysis is primarily due to patient factors. J Am Soc Nephrol 2016;28:645–652. [ Links ]
28. Quinn RR, Oliver MJ, Devoe D, Poinen K, Kabani R, Kamar F, et al. The effect of predialysis fistula attempt on risk of all-cause and access- related death. J Am Soc Nephrol 2017,28:613–620. [ Links ]
29. Ravani P, Quinn R, Oliver M, Robinson B, Pisoni R, Pannu N, et al. Examining the association between hemodialysis access type and mortality: the role of access complications. Clin J Am Soc Nephrol 2017;12:955–964. [ Links ]
30. Hall RK, Myers ER, Rosas SE, OHare AM, Colón-Emeric CS. Choice of hemodialysis access in older adults: a cost-effectiveness analysis. Clin J Am Soc Nephrol 2017;12:947–954. [ Links ]
Carla Leal Moreira, MD
Nephrology department, Centro Hospitalar Universitário do Porto,
Hospital Geral de Santo António
Largo Prof. Abel Salazar, 4099-001 Porto
E-mail: moreira.l.s.carla@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: Oct 2, 2017
Accepted in revised form: Nov 15, 2017














