Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.4 Lisboa dez. 2017
CASE REPORT
Cortical nephrocalcinosis asymmetrically involving the kidneys: a case report documenting the development via imaging
Ana Isabel Simões Ferreira, Natália Ferreira, João Leitão, José Fonseca-Santos
Radiology Department, Centro Hospitalar Lisboa Norte EPE; Lisbon, Portugal
ABSTRACT
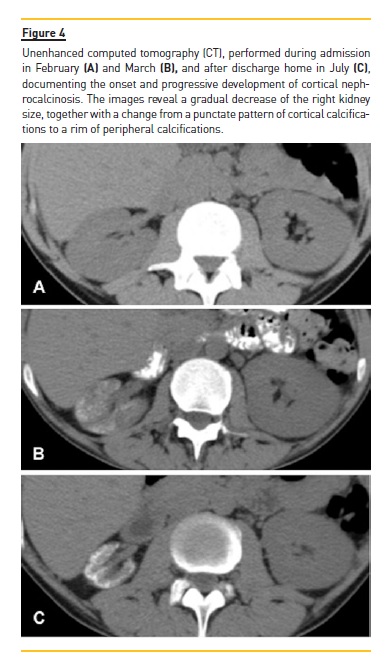
We report the case of a 38-year-old man with known human immunodeficiency virus infection who presented with severe Bordetella Bronchiseptica pneumoniae that led to septic shock and acute renal insufficiency, after which he developed bilateral cortical nephrocalcinosis, affecting most severely the right kidney with associated atrophy. The onset and progressive development of the findings were documented via both ultrasound and computed tomography. As far as we know, this is the first time that the development of cortical nephrocalcinosis has been documented via imaging, interestingly revealing a gradual decrease of the right kidney size, together with a change from a punctate pattern of cortical calcifications to a rim of calcifications.
Key-words: Acute cortical necrosis; acute renal insufficiency; Bordetella Bronchiseptica; cortical nephrocalcinosis; septic shock.
INTRODUCTION
Nephrocalcinosis consists of the generalized deposition of calcium (calcium oxalate or calcium phosphate) in the renal parenchyma, which can be either cortical or medullary in location. It is most commonly seen as an incidental finding, but it may be severe enough to cause renal failure1,2.
Cortical nephrocalcinosis is characterized by a peripheral location of the calcifications and is relatively uncommon; about 20 times less common than medullary nephrocalcinosis1,3. Interestingly, some clinical conditions may demonstrate combined cortical and medullary nephrocalcinosis (e.g. primary or secondary oxalosis, chronic pyelonephritis, intrarenal infection in HIV-seropositive patients)1.
CLINICAL CASE
We report the case of a 38-years-old man with previous history of human immunodeficiency virus (HIV) infection, routinely followed up by an infectious diseases physician at our hospital since 1997, with CD4+ count of 814.9 cells/uL and CD8+ of 1030 cells/uL, regularly medicated with Lopinavir, Ritonavir, Atazanavir and Raltegavir. Basal creatinine level was 0.63 mg/dL (0.7-1.3), glomerular filtration rate (GFR) 126 ml/min/1.73 (>90 ml/min/1.73) and the patient was not under vasopressor drugs. Between February and March 2016, he was admitted at the hospital with the diagnosis of a severe community-acquired pneumonia and Bordetella Bronchiseptica was isolated in sputum culture.
At the time of admission, the patient had a CD4+ count of 218 cells/μL and was initially medicated with Gentamicin, Ciprofloxacin, Piperacillin and Tazobactam.
Within one day after admission, the patient developed septic shock. In this setting, an acute renal insufficiency was installed two days after; creatinine level was 5.4 mg/dL, with an estimated glomerular filtration rate (GFR) of 12.4 ml/min/1.73 and urea 272 mg/dL (15-45mg/dL). Urine cultures were negative; urine tests showed pH of 6; increased erythrocytes 200 cel/uL; proteins 30 mg/dL and creatinine 100 mg/dL, as well as increased ratio protein/creatinine of 300 mg/g (<150 mg/g); calcium levels were normal; values of oxalate and pyridoxine were not evaluated. Gentamicin was interrupted. At that time, an ultrasound (US) demonstrated normal-size kidneys without hydronephrosis, but increased hyperechogenicity of most of the right renal parenchyma with loss of normal corticomedular differentiation (Fig. 1).
By May 2016, the renal function had returned to normal levels and the patient performed an US that showed a marked decrease in size of the right kidney, diffuse hyperechogenicity of the parenchyma with posterior acoustic shadowing suggesting the presence of parenchyma calcifications (Fig. 1). A CT was subsequently performed in July 2016 (Fig. 2 and Fig. 3) and confirmed an atrophy of the right kidney (6.5 cm of length) with a thin peripheral band or rim of cortical calcifications extending into the septal cortex, with sparing of the medullary pyramids, which retained the attenuation of the soft tissue.
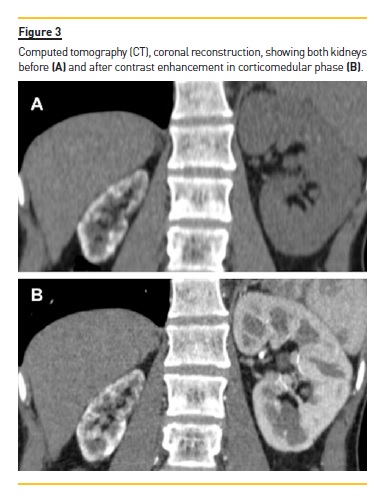
The left kidney was slightly enlarged (12.5 cm in length), but occasional punctate calcification randomly distributed in the cortex was also found. These findings were attributed to cortical nephrocalcinosis. CT angiography showed permeable kidney vessels, with a gradual and regular luminal decrease of the right renal artery, thought to be due to a decrease of the atrophic kidney needs. A retrospective analysis of previous examination found a thoracic CT performed during the course of the pulmonary infection, that allowed a partial view of the kidneys interested in the upper abdomen and documented a progressive increase in cortical nephrocalcinosis and a simultaneous progressive decreased in the right kidney size, from normal size to an atrophic kidney (Fig. 4).
DISCUSSION
Cortical nephrocalcinosis is an extremely rare finding. The most frequent causes are acute cortical necrosis and chronic glomerulonephritis4-8. But there are several other causes, such as intrarenal infection in HIV-seropositive patients by atypical mycobacterium or other organisms (e.g. Mycobacterium avium intracellular; Pneumocystis carinii; Hystoplasma; Cytomegalovirus), chronic pyelonephritis, Alport syndrome, vesicoureteral reflux, autosomal recessive polycystic kidney disease, acute or chronic transplant rejection, chronic hypercalcemia, hyperoxaluria, primary or secondary oxalosis, sickle cell disease, vitamin B6 (piridoxine) deficiency, nephrotoxic drugs (e.g. Amphotericin B, ethylene glycol, methoxyflurane)3,4,9.
In our case, the most likely predisposing factor for the development of cortical nephrocalcinosis was the acute cortical necrosis in the setting of septic shock after severe Bordetella Bronchiseptica pneumoniae. This is a common respiratory commensal of wild and domestic animals, rarely infecting humans. However, in immunosuppressed patients, especially in those with HIV infection, Bordetella Bronchiseptica can cause severe pulmonary infections10.
Although we cannot explain how a systemic cause could have affected both kidneys so differently, we did find a similar case in the literature, in which a cortical nephrocalcinosis of the right kidney was found after preeclampsia during pregnancy11. Another cause that was investigated was an intrarenal infection by atypical mycobacterium or other organisms in a HIV-seropositive patient. This seems less likely as no agent was identified in tests performed; furthermore, this infection tends to cause dystrophic calcification that asymmetrically involve the renal cortex and medulla, leading to combined cortical and medullary nephrocalcinosis12,13.
Renal cortical necrosis represents death of the renal cortex with sparing of the medulla, and is one of the most common causes of cortical nephrocalcinosis. It can result from any condition that causes an acute and prolonged shock14. Acute cortical necrosis may be seen in cases of sepsis, severe dehydration or bleeding, renal infarction or ischemia, haemolytic uremic syndrome; rejected renal transplant, extracorporeal shock wave lithotripsy, toxaemia of pregnancy, drugs, snake bite, arsenic poisoning3,4,11,15. The characteristic cortical calcification occurs within a few weeks after the onset of acute cortical necrosis. The medullary pyramids are usually spared, retaining the attenuation of the soft tissue. When cortical nephrocalcinosis first appears, the kidneys are still enlarged because of inflammatory oedema. With time, the kidneys become atrophic, as documented in the case we present.
Nephrocalcinosis found incidentally may be the presenting feature and the radiologist may be the first to suggest a diagnosis based on the pattern and distribution of renal parenchymal disease1. Radiographs may detect kidney calcifications depending on the size (if >2 mm), degree of increased attenuation (if on CT >100 HU), contrast factors and the spatial resolution of the film. Ultrasound (US) and computed tomography (CT) can detect nephrocalcinosis earlier than abdominal radiographs. US shows increased echogenicity of the renal cortex, which may simulate other medical conditions if acoustic shadowing is not present (acoustic shadowing depends on the degree of calcification).
MR is not a good imaging modality for calcifications detection, which appear as a signal void in both T1 and T2 weighted images4,16.
CT is considered the most sensitive and accurate imaging modality in depicting increased attenuation values in the basal and septal cortex with sparing of the medulla. It depicts nephrocalcinosis at an early stage and provides better imaging of the density and extent of the calcifications. Three patterns may be seen, although none is specific to any etiology: i) the most common pattern is a thin peripheral band or rim of calcification, often extending into the septal cortex; ii) a second pattern is two parallel lines of calcification, known as tram track appearance; these lines may be continuous, but more often they are interrupted, reflecting the more patchy distribution of cortical necrosis; iii) the least common pattern is punctate calcification distributed randomly in the cortex, representing necrotic calcified cortical glomeruli and tubules17.
The imaging signs of installation and development of cortical nephrocalcinosis, in US and CT, are interestingly depicted in the case we present. US was able to very soon (depict seven days after acute renal insufficiency was installed) a marked increase in parenchymal echogenicity of a normal-size kidney. On follow-up (three months later), the main features were an atrophic kidney with marked hyperechogenicity of the cortex and posterior acoustic shadowing (Fig. 1). CT performed at admission, one month and five months later, revealed a gradual decrease in right kidney size, together with a change from a punctate pattern of cortical calcifications to a rim of calcifications. On the other hand, on the upper pole of the left kidney which was affected to a much lesser degree and did not decrease in size, we found only scattered calcifications (Fig. 4). As far as we know, this is the first time that the development of cortical nephrocalcinosis has been documented via imaging and, maybe for that reason, we could not find literature describing a change in the cortical calcifications pattern. Looking at our images, it seems that the peripheral band of calcifications might have resulted from the convergence of the punctate calcification as a consequent of parenchyma atrophy. So, we can only speculate that in severe cases of diffuse nephrocalcinosis, the punctate pattern can evolve to a rim pattern of calcifications after kidney atrophy.
Nephrocalcinosis is caused by multiple different conditions and the renal prognosis is determined by the underlying cause. Whereas most patients with nephrocalcinosis do not progress to end-stage renal disease, certain underlying conditions, if not effectively treated, may be associated with progressive kidney dysfunction [2]. Although it is an irreversible condition when found, the importance of early detection relates with the ability of the clinician to closely follow-up renal function, undertake protective kidney measures and start a timely program for patients with end-stage renal disease.
References
1. Fulop T, Agraharkar M, Gupta R. Nephrocalcinosis. Emedicine Web site. http://emedicinemedscape.com/article/243911-overview. Updated Dec 07, 2015. Accessed July 30, 2017. [ Links ]
2. Kobrin SM, Curhan GC, Lam AQ. Nephrocalcinosis. UpToDate Web site. http://www.uptodate.com/contents/nephrocalcinosis. Updated Apr 07, 2017. Accessed July 30, 2017. [ Links ]
3. Knipe H, Gaillard F, et al. Cortical nephrocalcinosis. Radiopedia Web site. https://radiopaedia.org/articles/cortical-nephrocalcinosis. Accessed July 30, 2017. [ Links ]
4. Schepens D, Verswijvel G, Kuypers D, Vanrenterghem Y. Renal cortical nephrocalcinosis. Nephrol Dial Transplant (2000) 15: 1080-1082. [ Links ]
5. Wrong O. Nephrocalcinosis. In: Cameron S, ed. Oxford textbook of clinical nephrology. New York, Oxford University Press: 1998; 1380-1381. [ Links ]
6. Arons WL, Christensen WR, Sosman MC. Nephrocalcinosis visible by X-ray associated with chronic glomerulonephritis. Ann Intern Med 1955; 42: 260-282. [ Links ]
7. Cremin B, Wiggelinkhuizen J, Bonnici F. Nephrocalcinosis in children. Br J Radiol 1982; 55: 413-418. [ Links ]
8. Harris L, Cohen E, Kassner G, Haller JO. Nephrocalcinosis in chronic glomerulonephritis: report of the youngest patient. Urol Radiol 1980; 2: 51-52. [ Links ]
9. Dyer R B, Chen M Y M, Zagoria R J. Abnormal calcifications in the urinary tract. Radiographics 1998; 18: 1405-1424. [ Links ]
10. Rampelotto RF, Hörner A, Hörner C, Righi R, Hörner R. Pneumonia caused by Bordetella bronchiseptica in two HIV-positive patients. Sao Paulo Med. J. vol. 134 no.3 São Paulo May/June 2016.
11. Lang EK, Aberg C, Kagen A, Macchia R J. Cortical Nephrocalcinosis in a Patient with History of Preeclampsia. Journal of Urology; Vol. 179, 1577, April 2008. [ Links ]
12. Symeonidou C, Standish R, Sahdev A, Katz RD, Morlese J, Malhotra A. Imaging and histopathologic features of HIV-related renal disease. RadioGraphics 2008. [ Links ]
13. Falkoff G, Rigsby CM, Rosenfield AT. Partal, Combied cortical and medullary nephrocalcinosis: US and CT patterns in AIDS-associated MAI Infection. Radiology 1987; 162:343-344. [ Links ]
14. Lee H J. Nephrocalcinosis. In: Kim SH. Radiollogy illustrated: uroradiology, Springer-Verlag Berlin Heidelberg, 2012; 529-530. [ Links ]
15. Calvino J, Romero R, Blanco M, Lens XM, Novoa D, Sanchez-Guisande D. Cortical nephrocalcinosis induced by extracorporeal shock wave lithotripsy. Nephron 1999; 81:242-243. [ Links ]
16. Manz F, Jaschke W, Van Kaick G, Waldherr R, Willich E. Nephrocalcinosis in radiographs, computed tomography, sono-graphy and histology. Pediat Radiol 1980; 9: 19-26. [ Links ]
17. Banner MP. Nephrocalcinosis. In: Pollack HM, ed. Clinical urography. WB Saunders Company 1990: 1768-1769. [ Links ]
Ana Isabel S. Ferreira, MD
Av. Prof. Egas Moniz, 1649-035 Lisboa, Portugal
Email: anaisabelvnsf@gmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: Aug 15, 2017
Accepted in revised form: Oct 30, 2017














