Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.4 Lisboa Dec. 2017
CASE REPORT
72-year-old man with acute kidney injury, hypercalcemia and metastatic prostate câncer
Alice Lança, Paulo Santos, Francisco Ferrer
Department of Nephrology, Centro Hospitalar Médio Tejo, Torres Novas, Portugal
ABSTRACT
Paraproteinemias are characterized by the abnormal expansion of a plasma cell clone with overproduction of a monoclonal (M) immunoglobulin. In rare cases (1%) two distinct M proteins can be identified (biclonal gammopathy).
Renal manifestations are frequent and can present with several histological patterns. Prognosis and treatment are similar to monoclonal gammopathies varying according to extent of disease and response to therapy. We report a case of a 72-year-old man with a prior history of hypertension, dyslipidemia, and prostate cancer with bone metastasis under treatment with leuproline, cyproterone, and nonsteroidal anti-inflammatory drugs who was found to have anemia, acute kidney injury, and hypercalcemia. After clinical evaluation and workup, a biclonal multiple myeloma (IgG kappa and IgA lambda) and a cast nephropathy were diagnosed. The patient was started on renal replacement therapy and on CyBorDex treatment cycle protocol for two years with remission of multiple myeloma but without renal function recovery. During this period, there was no prostate cancer progression. This case report alerts us to the rarity of a biclonal multiple myeloma especially in a patient with advanced prostate cancer but also to the fact that not all osteolytic lesions are secondary lesions.
Keywords: acute kidney injury, biclonal gammopathy, cast nephropathy, hypercalcemia, multiple myeloma, prostate câncer
INTRODUCTION
Paraproteinemias are a heterogeneous group of disorders characterized by clonal proliferation of abnormal plasma cells with overproduction of a monoclonal (M) protein in a form of the whole immunoglobulin or a fragment (heavy or light chain alone)1. Biclonal gammopathy is defined as the simultaneous appearance of two distinct M components in serum and/or urine corresponding to approximately 1% of all gammopathies2.
This may result from a neoplastic transformation of two distinct malignant plasma cell lines or from the production of two independent proteins from a single clone of B-lymphoid cells3. The most common combinations are IgG and IgA followed by IgM and IgG, which may be further divided depending on the type of heavy or light chain detected4. Kidney dysfunction in multiple myeloma (MM) is a common complication, along with hypercalcemia, anemia, and lytic bone lesions. Cast nephropathy is the most frequent pathologic lesion associated with MM. It results from free light chain (FLC) deposition, leading to tubular obstruction, atrophy, and interstitial inflammation with giant cell infiltration, causing irreversible damage and progression to renal failure5. The presence of kidney disease has a very important prognostic value due to its significant morbidity and mortality.
We report a rare case of MM with biclonal gammopathy (IgG kappa and IgA lambda) in a 72-year old man with prostate cancer who presented with bone pain, anemia, and renal failure.
CASE REPORT
A 72-year-old Caucasian male presented to the emergency department with generalized bone pain, weakness, and fatigue. Approximately 1 year before this presentation, the patient had received a diagnosis of prostate adenocarcinoma with multiple skeletal lesions in the bone scan and was started on gonadotropinreleasing hormone agonist (Leuprolide) and on nonsteroidal anti-inflammatory drugs (NSAIDs) on a regular basis for pain management. In addition, his past medical history included systemic hypertension, dyslipidemia, and chronic normocytic normochromic anemia, but no kidney disease. Medications included simvastatin 20mg, amlodipine/valsartan 5mg/80mg and cyproterone 100mg daily. He had no known allergies. On examination, his temperature was 36.7°C, heart rate 99 beats per minute, blood pressure 187/93 mmHg, oxygen saturation 98% while patient was breathing ambient air, and his urinary output was 0.71 mL/kg/hr (800mL/16hours). He appeared mildly ill with marked pallor and and dehydrated mucosa, cachexia, and an ejection systolic heart murmur on cardiac auscultation.
The remainder of the examination was normal. Laboratory evaluation revealed hemoglobin: 6.7 g/dL, white blood cells: 9000/uL with normal neutrophil leukocyte count, thrombocytopenia: 75 000 x 10^9/L, creatinine: 13.4 mg/dL, urea: 204 mg/dL, albumin: 3.6 g/dL, calcium: 13.2 mg/dL, phosphorus: 6.5 mg/dL, parathyroid hormone (PTH): 302 pg/mL, vit D: 13.8 ng/mL (>20 ng/mL), C-reactive protein (CRP): 0.13 mg/dL and prostate specific antigen (PSA): 4 ng/mL. Peripheral blood smear showed anisocytosis and spiculated cells (burr cells).
Urinalysis was only positive for proteinuria (30 mg/dL) with inactive sediment, but the 24-hour protein collection detected 2.15g. Renal ultrasound (RUS) showed normal-sized kidneys (left kidney 12.3 cm and right kidney 11.5 cm) with undifferentiated renal parenchyma and increased echogenicity. No signs of obstruction or lithiasis were seen. Despite the fact the patient was started on vigorous intravenous hydration and given blood transfusions to correct hypovolemia, urinary output declined and renal function worsened, so he was started on hemodialysis (HD). As part of the study of the clinical picture of anemia, renal failure, and hypercalcemia, additional imaging studies were obtained. Chest radiography was normal but the skull X-ray showed lytic lesions in salt and pepper appearance (Fig. 1) and the bone scan revealed increased uptake in posterior third of the 10th left, 5th and 11st right costal arches, left ischiopubic arch, anterior inferior portion of acetabulum, and in the right orbital arch.
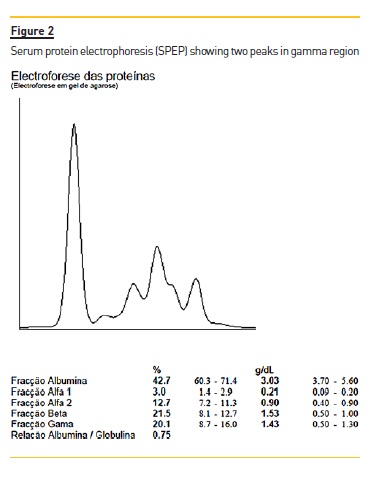
Serum protein electrophoresis (SPEP) revealed two M spikes (Fig. 2) and immunofixation showed a biclonal profile (IgG kappa and IgA lambda). IgG level was 9.65 g/L (reference range: 7.51–15.6), IgA levels were 15.60 g/L (reference range: 0.82–4.53) and IgM 0.12 g/L (reference range: 0.46–3.04). Serum free light kappa chain levels were 10.3 g/L (reference range: 6.29–13.5) and lambda 9.32 g/L (reference range: 3.13–7.23) with a free light ratio kappa/lambda of 1.11. Bence Jones protein (free kappa and lambda light chains) was detected in the urine. Urinary FLC kappa were 0.385 g/L (reference range: <0.018) and lambda 3.13 (<0.005). Free kappa/lambda light chain ratio was 0.09 (0.26-1.65).
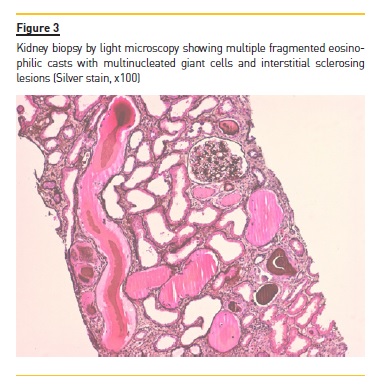
Angiotensin-converting enzyme (ACE) was negative. Kidney biopsy showed several fragmented casts surrounded by multinucleated giant cells involving 70% of tubular interstitium with sclerosing lesions (Fig. 3).
On immunofluorescence (IF), the casts were both kappa and lambda light chains. Bone marrow biopsy revealed 40% plasma cells infiltration including binucleate forms, pointing towards a biclonal multiple myeloma. Based on these findings, the diagnosis was discussed with the hematological team. The patient was started on CyBor-Dex treatment cycle protocol which included the administration of bortezomib 2.2 mg (1.3mg/m2) subcutaneously every 72/72h for 8 cycles (3 administrations per 21 days), cyclophosphamide 100 mg (300 mg/m2) twice a week orally after HD and finally dexamethasone 8 mg 5 consecutive days per week after HD. Prophylactic aciclovir 200 mg bd to tid and nystatin daily was given for the duration of treatment and 3 months post therapy. Two years and 8 cycles (24 doses) of chemotherapy after the initial diagnosis, immunoglobulins levels significantly dropped to normal levels. Unfortunately, there was no renal function recovery and the patient remained dialysis dependent. His prostate cancer remained stable after stopping the treatment with gonadotropin-releasing hormone agonist. Recently, his PSA is 0.8 ng/mL.
DISCUSSION
In this case report, a 72-year-old man with a prior history of hypertension, dyslipidemia, and prostate cancer with bone metastasis under treatment with androgen deprivation therapy (ADT) and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to have anemia, acute kidney injury (AKI), and hypercalcemia.
Prostate cancer is the second most frequently diagnosed cancer in men and typically presents with anemia and bone pain, with or without pathologic fracture, among other features. The axial skeleton, the pelvis, and the retroperitoneum are the most frequent sites of metastasis6. Bone lesions are predominantly osteosclerotic and rarely osteolytic, stimulating tumor growth and progression7. They represent a major cause for morbidity, characterized by severe pain, impaired mobility, pathologic fractures, spinal cord compression, bone marrow aplasia, and hypercalcemia. The patient had been receiving ADT for symptomatic relief for the past year. Of particular concern was the regular use of NSAIDs for pain management. Both these medications, especially the latter, interfere with renal function and may cause azotemia. Additionally, given the history of metastatic prostate cancer to the bone, hypercalcemia could induce AKI. Consequently, oliguric renal failure secondary to hypercalcemia, volume depletion, and drug-induced nephrotoxicity from NSAIDs and ADT, create a plausible diagnosis. Cancer in particular, accounts for 35% of cases of hypercalcemia through production of parathyroid hormone–related protein (PTHr) in many solid tumors, excess production of 1,25-dihydroxyvitamin D in some patients with lymphoma, and osteolytic bone lesions in persons with multiple myeloma or cancer that metastasizes to bone.
Skeletal metastases account for the majority of all malignant bone tumors, and are seen in a vast number of primary cancers, although lung cancer, breast cancer, renal cell carcinoma, and prostate cancer account for approximately 80% of all cancers. However, the subnephrotic proteinuria, worsening renal function with urinary output decline, and the need for HD (even after nephrotoxic drugs were withheld and vigorous hydration was started) did not support this diagnosis. Furthermore, the hemoglobin level and the platelet count were too low and continued to drop despite blood transfusions.
To determine the cause of these findings, it was necessary to consider disorders associated with anemia and hypercalcemia that involve the kidney and cause systemic symptoms. Tubulointerstitial diseases, including multiple myeloma and granulomatous diseases, such as tuberculosis and sarcoidosis as well as granulomatosis with polyangiitis and tumors, can easily cause anemia, raise serum calcium, and worsen renal function.
Regarding sarcoidosis and vasculitis, there were no findings to support neither of these diagnoses; ACE and ANCA were both negative, Vit D level was low, chest radiographs were clean (absence of pulmonary interstitial infiltrates or nodules) and there were no cutaneous lesions. Nonetheless, the combination of severe anemia, persistent hypercalcemia, bone pain and kidney disease requiring hemodialysis in a 72-yearold male patient, were suggestive of MM. Hence, a full hematological workup including cranial and long bones X-ray and a bone marrow biopsy were requested. Positive findings such as salt and pepper appearance typical of lytic lesions together with altered electrophoresis and immunofixation were determinant for the diagnosis of biclonal MM. Renal biopsy corroborated this diagnosis by showing cast nephropathy.
The diagnosis of myeloma often results from the workup of unexplained proteinuria and renal insufficiency.
However, it was not straightforward as there were several confounding and precipitating factors to justify the clinical picture. Immunological study, bone and kidney biopsy were essential to confirm the diagnosis.
MM is a B cell-derived malignancy that results in increased production of monoclonal immunoglobulins (Ig) and bone destruction. With this in mind, we can suspect that bone lesions at the time of the diagnosis of prostate cancer could actually be osteolytic lesions from MM rather than secondary lesions. Bone metastases are almost always multiple and involve axial skeleton but MM lytic lesions can be numerous too, wellcircumscribed and despite occurring predominantly in the thoracic and lumbar spine (vertebrae) they can also be encountered in the axial skeleton. Although the diagnosis could not be made solely on the distribution of the bone lesions, their lytic nature, the presence of symptomatic high serum calcium, high PTH and signs of chronic kidney disease on the ultrasound and renal biopsy further support this view and exclude pathologies such as primary hyperparathyroidism.
Biclonal gammopathy occurs when two distinct M components are present in serum and/or urine; they arise either from independent proliferation of two distinct plasma cell clones or from a single clone of B-lymphoid cells producing two different monoclonal proteins3.
These are usually more symptomatic than monoclonal gammopathies even though they do not differ in respect to clinical features, presentations, response to therapy or outcome. Also, in contrast to monoclonal gammopathies, they are more frequently associated with lymphoproliferative disorders (mostly Waldeströms macroglobulinemia) compared to MM or other non-hematological disorders (1.2%). As presented in this case, true biclonal myeloma expressing both kappa and lambda are not exceptional but quite rare (1-2%). Kidney involvement occurs in 50% of MM and usually requires immediate start of dialysis (10%) and chemotherapy for clinical improvement and prolonged survival8. The decision to initiate treatment depends on whether there is evidence of organ or tissue impairment (end organ damage) manifested by anemia, hypercalcemia, lytic bone lesions, renal insufficiency, hyperviscosity, amyloidosis, or recurrent infections. The initial therapy consists of aggressive hydration, urine alkalinization, chemotherapy (dexamethasone alone or in combination with cyclophosphamide, thalidomide, vincristine, doxorubicin), and autologous stem cell transplant (ASCT) which increases overall median survival in 12 months. However, ASCT is not frequently proposed for patients over 65 years of age due to discordant results9.
As previously mentioned, immediately after the diagnosis of MM, the patient was started on chemotherapy (cyclophosphamide) and high-dose dexamethasone which induces rapid apoptosis and lowers light chain (LC) concentration. Bortezomib, by inhibiting proteasome, leads to massive apoptosis of plasma cells, reducing tumoral burden and monoclonal protein production, thus leading to a significant reduction of serum monoclonal components. According to the hematology team, based on the patients age and severe comorbidities (renal failure on hemodialysis), ASCT was not an option. Plasma exchange was also not performed as it didnt seem likely to improve either recovery from renal failure or patient survival10.
Although some conflicting results have demonstrated the potential of high cutoff dialysis membrane in reversing renal failure in patients with cast nephropathy, it is only when the diagnosis of MM is promptly made and bortezomib is immediately started. In this case report, neither of these criteria were met; both the diagnosis and the referral to the Hematological Unit were delayed. Moreover, the amount of fibrosis and sclerosing lesions on the renal biopsy represented well-established features of chronicity. For all these reasons, conventional hemodialysis was performed on an intermittent basis11,12. Unfortunately, the patient showed no signs of renal function recovery and remained dialysis-dependent. To the best of our knowledge, patients requiring dialysis have reported rates of recovery of renal function as low as 5–15%13. In regards to prostate adenocarcinoma, that there was no progression after suspension of therapy lead us to believe that myeloma was most probably already in progression at the time of the diagnosis of prostate cancer, but was camouflaged.
This case is particularly interesting because it illustrates how two different diseases may have very similar clinical pictures. Also, the rarity of a biclonal MM should be highlighted as a rare form of MM. All things considered, we can conclude by saying physicians should always have in mind that things arent always what they seem.
References
1. Chen ZW, Kotsikogianni I, Raval JS, Roth CG, Rollins-Raval MA. Biclonal IgD and IgM plasma cell myeloma: a report of two cases and a literature review. Case Rep Hematol [Internet]. 2013;2013:293150. [ Links ]
2. Gu HJ, Cho SY, Lee J, Sung JY, Park TS. Biclonal plasma cell myeloma with the simultaneous appearance of both secretory lambda and nonsecretory kappa monoclonal light chains. Clin Chem Lab Med. 2017;55(1):e21–4. [ Links ]
3. Srinivasan VK, Bhagat P, Bansal F, Chhabra S. Occurrence of double monoclonal bands on protein electrophoresis: an unusual finding. Indian J Hematol Blood Transfus. 2016;32(1):184–8. [ Links ]
4. Mahto M, Balakrishnan P, Koner BC, Lali P, Mishra TK, Saxena A. Rare case of biclonal gammopathy. Int J Case Reports Images [Internet]. 2011;2(2):11. [ Links ]
5. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med [Internet]. 2011;364(11):1046–60. [ Links ]
6. Bhattar R, Maheshwari A, Singh Yadav S, Tomar V. Unusual presentation of prostate carcinoma: a case report. J Clin Diagnostic Res [Internet]. 2017;11(2):6–7. [ Links ]
7. Zelmann R, Mari F, Jacobs J, Zijlmans M, Dubeau F, Gotman J. Pub Med Central CANADA. Clin Neurophysiol. 2013;123(3):106–16. [ Links ]
8. Ecotière L, Thierry A, Debiais-Delpech C, Chevret S, Javaugue V, Desport E, et al. Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dial Transplant. 2016;31(1):64–72. [ Links ]
9. Mohty M, Harousseau J-L. Treatment of autologous stem cell transplant-eligible multiple myeloma patients: ten questions and answers. Haematologica [Internet]. 2014;99(3):408–16. [ Links ]
10. Clark WF, Stewart AK, Rock GA, Sternbach M, Sutton DM, Barrett BJ, et al. Plasma exchange when myeloma presents as acute renal failure. Ann Intern Med. 2005;143(11):777–84. [ Links ]
11. Finkel K, Fabbrini P. High cut-off hemodialysis for myeloma cast nephropathy – do we finally have an answer? J Onco-Nephrology [Internet]. 2017;1(2):67–70. [ Links ]
12. Hutchison CA, Harding S, Mead G, Goehl H, Storr M, Bradwell A, et al. Serum free-light chain removal by high cutoff hemodialysis: optimizing removal and supportive care. Artif Organs. 2008;32(12):910–7. [ Links ]
13. Hutchison CA, Bradwell AR, Cook M, Basnayake K, Basu S, Harding S, et al. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin J Am Soc Nephrol. 2009;4(4):745–54. [ Links ]
Alice Lança, MD
Department of Nephrology, Centro Hospitalar Médio Tejo
Av. Xanana Gusmão, 2350-754 Torres Novas, Portugal
Email: alicelancabaptista@gmail.com
ACKNOWLEDGMENTS
The authors would like to take this opportunity to thank Dr. Maria Fernanda Carvalho (Kidney Morphology Laboratory, Department of Nephrology, Hospital Curry Cabral, Lisbon, Portugal) for her availability and dedication in sharing and discussing the histopathological renal biopsy specimens with us.
Disclosure of potential conflicts of interest: none declared
Received for publication: Oct 25, 2017
Accepted in revised form: Dec 20, 2017














