Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Portuguese Journal of Nephrology & Hypertension
versión impresa ISSN 0872-0169
Port J Nephrol Hypert vol.33 no.4 Lisboa dic. 2019
https://doi.org/10.32932/pjnh.2020.01.044
ORIGINAL ARTICLE
Henoch-Schönlein Purpura: What to expect
Maria João Gaia, Mariana Capela, Joana Pires Borges, Eduarda Marques, Graça Ferreira, António Vinhas da Silva
Department of Pediatrics / Neonatology, Centro Hospitalar de Vila Nova de Gaia/Espinho; Portugal
ABSTRACT
Introduction: Henoch-Schönlein Purpura is the most common systemic small vessel vasculitis of childhood. It is most frequently a selflimited entity, although some cases with concomitant nephritis may have serious complications. This study aims to analyze the clinical, epidemiologic and prognostic factors of children with this disease. Methods: Observational and retrospective study of children with diagnosis of Henoch-Schönlein Purpura from January 2011 to June 2017. Results: 61 patients were identified, with a median age of 6 years and a slight predominance of females. Purpura was present in all cases; the second most common symptom was arthralgia (75.4%), followed by gastrointestinal (39.3%), renal (31.1%) and scrotal involvement (6.6%). Corticotherapy was used in 12 patients (19.7%). Almost one fourth of the patients had a recurrence, but only 4 had persistent manifestations for longer than 6 months. Abdominal symptoms were found to have a positive correlation with renal involvement and corticotherapy showed no protective long-term effects. Discussion: Henoch-Schönlein Purpura is mostly a self-limited pathology. We found no association between age or sex and a poorer outcome, but a correlation between abdominal and renal involvement was found. We found a negative correlation between joint involvement and recurring episodes, showing that it may be a good prognostics factor. Corticotherapy demonstrated no protective long-term effect, and was on the contrary correlated with hospitalization, recurrence and persistence of signs and/or symptoms. This correlation can be explained by the fact that patients having indication for and receiving corticotherapy had an inherently more severe spectrum of the disease and so were more prone to poorer outcomes.
Key words: Corticosteroids, Henoch-Schönlein, Purpura, Vasculitis
INTRODUCTION
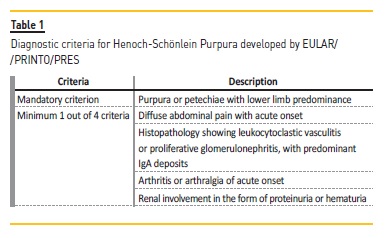
Henoch-Schönlein purpura (HSP) is the most common childhood vasculitis.1 It is a systemic IgA-mediated leukocytoclastic small-vessel vasculitis, that typically presents with a classic tetrad of features including palpable purpura, arthritis or arthralgia, gastrointestinal complaints and renal disease.2,3 It has an estimated incidence of 10-20 children per 100,000 per year.1,4 A male predominance of 1.2-1.5:1 has been described2,5, although other studies have found equal predominance between males and females.6 Most of the cases occur in children less than 10 years old.4,6,7 HSP is most commonly a self-limited disease with excellent prognosis, although some cases with concomitant nephritis may have serious complications.1,6 The diagnosis is based on clinical criteria developed by EULAR/PRINTO/PRES (European League Against Rheumatism/Paediatric Rheumatology International Trials Organisation/Paediatric Rheumatology European Society), presented in 2008, with sensitivity of 100% and specificity of 87% (Table 1). These criteria include a mandatory criterion of purpura or petechiae with lower limb predominance with a minimum of one additional criterion from the following: diffuse abdominal pain with acute onset, histopathology showing leukocytoclastic vasculitis or proliferative glomerulonephritis with predominant IgA deposits, arthritis or arthralgia of acute onset or renal involvement in the form of proteinuria or haematuria.8 The treatment, which is usually symptomatic, may sometimes demand the use of steroids or other immunomodulators, depending on the severity and organ involvement.1
The aim of this study was to analyze the clinical, epidemiologic and prognostic factors of children with this diagnostic in our institution.
METHODS
The authors institution is a level II Hospital, with a hospital catchment area serving 334 081 inhabitants (population of Vila Nova de Gaia and Espinho), 61 225 of them in the pediatric age range. This was an observational longitudinal retrospective study based on information collected from the clinical records of children with the diagnosis of HSP who met the EULAR/PRINTO/PRES criteria, from the Emergency Room or Inpatient Service of our Institution. The data was collected from January 2011 to June 2017. Exclusion criteria included children with previous renal pathology or not presenting with a first episode of HSP.
The following variables were studied: age, sex, potential triggers, signs/symptoms and time of presentation, need for biopsy, need for hospitalization, treatment, follow-up, recurrence and persistence of signs/symptoms.
The standard follow-up was defined as one year, given the fact that about 90% of children who develop renal involvement do so within two months of diagnosis, and 97% within six months.9 The standard follow-up consisted in weekly clinical appointments with urinary dipstick and blood pressure measurements in the first month, followed by biweekly appointments for the next two months, monthly appointments until six months, and a last appointment one year after the diagnosis. The follow-up was individualized in specific cases, and we had a mean follow-up of 2.2 years (minimum 1 year, maximum 7.8 years) in cases with renal involvement.
Statistical analysis, including descriptive statistics and inferential statistics, was performed using IBM SPSS Statistics version 23. Categorical variables are presented as absolute or relative frequencies and continuous variables as means and standard deviations if normally distributed, and median and interquartile range if non-normally distributed.
Normal distribution was evaluated using the Kolmogorov-Smirnov test or through analysis of skewness and kurtosis. Independent T-test and Mann-Whitney test were used to assess association between age and multiple follow-up variables. To identify risk factors related to the prognosis, the authors used the Chi-square test and Fishers exact test. All reported p values are two-tailed, with a value <0.05 indicating statistical significance.
RESULTS
The sample included 61 patients with the diagnosis of HSP, with a slight predominance of females (n=33; 54.1%) and a median age of 6 years (minimum 6 months, maximum 16 years). The incidence of HSP was 15.3/100000. There was a precedent upper airway infection in 32 cases (52.5%).
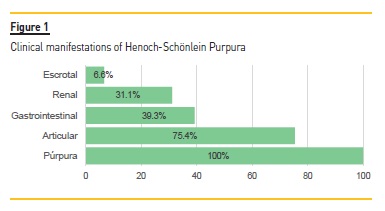
Purpura was present in all cases. The second most common symptom was arthralgia (n=46; 75.4%), followed by gastrointestinal symptoms (n=24; 39.3%), renal involvement (n=19; 31.1%) and scrotal involvement (n=4; 6.6%). Purpura was one of the presenting signs in 52 cases (85.2%), with the remaining 9 cases having as first symptom arthralgia (n=6; 9.8%) and abdominal complaints (n=3; 4.9%) (Fig. 1).
The most affected joint was the ankle (n=35; 57.3%), followed by the knee (n=17; 27.9%), wrist (n=10; 16.4%), toes (n=3; 4.9%), fingers (n=3; 4.9%) and elbow (n=1; 1.6%). The majority had monoarticular complaints (n=26; 42.6%), with the rest having oligoarticular involvement, in up to 3 joints. The articular symptoms appeared before the purpura in 6 cases (9.8%), at the same time in 27 (44.3%) and afterwards in 13 (21.3%).
Almost all of those presenting abdominal complaints reported colicky abdominal pain (n=23; 37.7%), and also including vomiting (n=10, 16.4%), diarrhea (n=2; 3.3%) and gastrointestinal bleeding (n=2; 3.3%). Almost one fourth of the patients were submitted to abdominal ultrasonography (n=14; 23%), with 3 of them having altered results (mesenteric adenopathy (n=2), colic wall thickening (n=1) and pelvic fluid (n=1). Abdominal complaints preceded purpura in 3 cases (4.9%), occurring at the same time in 7 (11.5%), and after in 14 (23%).
A serum creatinine measure was performed in 95.1% of patients at admission, with a mean value of 0.36mg/dl (minimum 0.16mg/dl, maximum 0.86mg/dl). All patients were submitted to a urine dipstick at admission, with 12 of them having altered results (19.7%), including isolated proteinuria (n=7; 11.5%), isolated microhematuria (n=4; 6.6%) and hematuria with proteinuria (n=1; 1.6%). During follow-up, 9 patients (14.8%) developed new manifestations, such as microhematuria (n=3; 4.9%), proteinuria (n=6; 9.8%) and macrohematuria (n=2; 3.3%). These occurred during normal evolution in 6 patients (9.8%) and in clinical recurrences in 3 patients (4.9%). In the patients with proteinuria, the median value in the urine dipstick was 1+ (minimum 1+, maximum 3+), with 3 patients having quantified proteinuria through 24 hour urine protein test (one case with maximum 88 mg/m2/day, one with 7 mg/ m2/day and one with 6.4 mg/ m2/day) or urinary protein/creatinine ratio (one case with maximum 2.4 mg/mg).
Hematuria was quantified in 4 cases through urine sediment examination, with values between 24 and 250 erythrocytes/μl. There were no cases of altered kidney function during follow-up. There was one case of acute renal injury (1.6%), two cases of hypertension (3.2%) and two cases of nephrotic proteinuria (3.3%).
The case of acute kidney injury was of a 6-year-old boy who had normal serum creatinine value, blood pressure and urine dipstick at presentation, but returned to the Emergency Room 4 days after due to worsening of the purpuric rash. Another blood test was performed, with a creatinine value of 0.86mg/dl and systolic blood pressure above the 95th percentile. The kidney ultrasound showed signs of medical nephropathy and the child was admitted to initiate corticotherapy (methylprednisolone 30 mg/kg/day in bolus). The serum creatinine normalized after the first bolus and the blood pressure was normal after the 2nd day of hospitalization. There were no alterations during follow-up and he continued with oral corticotherapy for 3 months (prednisolone in reduction scheme). The other case of hypertension was of an 8-year-old boy who presented with systolic blood pressure above the 95th percentile in the Emergency Room, along with proteinuria, but who presented normal blood pressure values throughout the follow-up.
Of all the patients with renal involvement, 11 presented it along with the purpura appearance (18%), and 8 presented it for the first time only afterwards (13.1%) (Table 2). No difference was found between the 2 groups in terms of age, gender, mean creatinine and proteinuria and need for corticotherapy. The timing of renal involvement had a median of 2 weeks from presentation (minimum 0 days, maximum 6 months), and lasted for a median of 2 months (minimum 1 week, maximum 4 years).
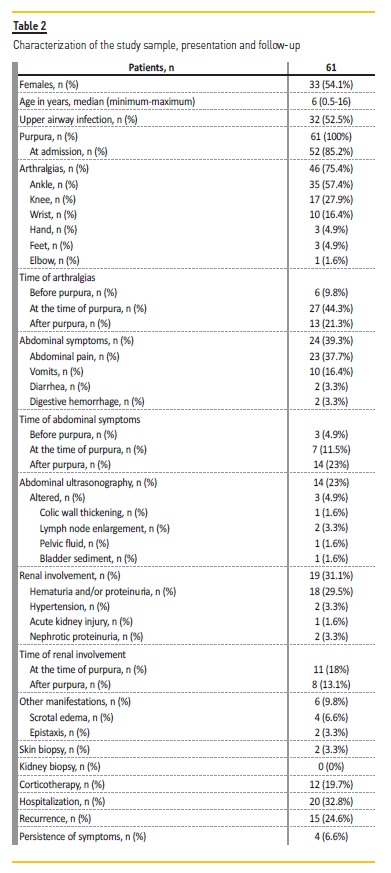
There was need for hospitalization in 20 cases (32.8%), with a mean duration of 2.7 days. The causes for hospitalization were arthralgias with functional incapacity (n=9; 14.8%), inadequate oral intake (n=5; 8.2%), 2 of them with gastrointestinal bleeding, marked scrotal involvement (n=4; 6.6%), acute kidney injury (n=1; 1.6%) and for surveillance and intravenous antibiotherapy for cellulitis (n=1; 1.6%). Corticotherapy was used in 12 patients (19.7%), 10 of them intravenously (16.4%). The causes for corticoid administration were marked scrotal involvement (n=4; 6.6%), arthritis with functional incapacity (n=3; 4.9%), acute kidney injury with ultrasound signs of nephropathy (n=1; 1.6%), abdominal pain and gastrointestinal bleeding with no improvement (n=1; 1.6%), persistent and worsening hematoproteinuria after 1 month (n=1; 1.6%), persistent abdominal pain and purpuric rash after 1 and a half months (n=1; 1.6%) and due to family history of Henoch-Schönlein purpura with kidney failure and kidney transplant (n=1; 1.6%). The schemes performed in the patients with renal involvement were both 30 mg/kg/day bolus of methylprednisolone for 3 days, followed by prednisolone in reduction scheme. The timing of initiation of corticoid had a median of 7 days (minimum 1 day, maximum 45 days).
Treatment with nonsteroidal anti-inflammatory drugs was administered in 90.6% of cases and in 1 case an ACE inhibitor was prescribed due to persistent proteinuria. No other immunosuppression schemes were used. Skin biopsy was performed in 2 cases (3.3%) for diagnostic confirmation, and no kidney biopsies were performed.
During follow-up, 15 patients (24.6%) presented at least one recurrence, but only 4 (6.6%) had persistent manifestations for longer than 6 months. One patient had persistent proteinuria for 2 years, in the nephrotic range for the first 4 months and associated with hematuria in the first 2 months, with an episode of macrohematuria. He had a normal renal function throughout the follow-up. Another patient had persistent hematuria for 4 years after diagnosis, maximum 2+ in the urine dipstick and 70 erythrocytes/μl in the urine sediment examination, with normal renal function. The third patient was under corticotherapy from admission due to family history of Henoch-Schönlein purpura with kidney failure and kidney transplant in her brother. She had persistent purpuric rash for 18 months, that worsened in the first 3 months, granting a skin biopsy for diagnostic confirmation, that had a normal result (a repetition due to the long-term corticotherapy was suggested). The adolescent had proteinuria (maximum 7mg/m2/day) for 9 months. The last case was of a patient who developed hematuria 6 months after diagnosis that persisted for 4 years, with a maximum of 250 erythrocytes/μl. He also had proteinuria (maximum 6.4 mg/m2/day) until 15 months after diagnosis. The other patient who was subjected to a skin biopsy had a persistent purpuric rash for 4 months, and the result was compatible with an evolved phase of leukocytoclastic vasculitis.
A negative correlation was found between joint involvement and the presence of a recurrent episode (p=0.034). Abdominal complaints had a positive correlation with renal involvement (p=0.002). No correlation was found between age or sex and renal involvement or severity. No protective effect on the outcome was demonstrated for corticotherapy. No correlation was found between the use of steroids and renal involvement, and renal involvement was indeed correlated with hospitalization, recurrence and persistence of manifestations (p<0.05).
DISCUSSION
HSP is a somewhat common diagnosis in childhood, with reported incidence rates in the pediatric population between 10 and 20/100000.
The incidence rate found in our study falls into this interval. The majority of cases are self-limited2,5,6, which is consistent with our findings of only 6.6% of the patients having persistent manifestations for longer than 6 months. Recurrence is common, described in literature as being 30-40%5,6,10,11, and found in our study in almost one fourth of the patients. Several risk factors for recurrence have been described, including children older than 8 years and renal involvement12, which we have not found in our analysis.
It is a much more common diagnosis in children younger than 10 years, with mean age 6 years old, as found in the great majority of literature on this subject.1-7 There have been some studies describing equal incidence between genders, similar to what we have found.6
There was an upper airway infection in more than half of the cases, consistent with it being the most commonly pointed trigger.6,10,13-15,18 The signs and symptoms can develop in different orders, with the most common presentation purpura and arthralgias, as we have found.1 Articular involvement has been described in 74-82% of the cases5,6,16, abdominal complaints in 50-75%5,13,17-20, renal involvement in 20-60%5,9,16,20 and urogenital involvement in boys up to 27%5,21, consistent with the incidences we have found.
We have found that in 9.8% and 4.9% of patients the first symptom was arthralgia and abdominal complaints, respectively. This is supported by the described incidence of joint pain as first symptom of 15%16 and abdominal symptoms from 10-40%16,22-24, before the appearance of purpura.5,17
The most affected joints are the lower limbs (ankles and knees), with wrists, hands and feet being affected as well, with mono or oligoarticular involvement, up to 3 joints.5,6 In terms of abdominal complaints, we found abdominal pain to be the most common one, as is often described13,18-20, with gastrointestinal bleeding being rare, and no serious complication, such as intussusception, intestinal infarction or perforation, occurring. This is supported by whats described in literature, with hemorrhage not being common, but occurring in up to 22% in some studies, and intussusception being a rare complication in less than 3% of cases.2,20
Renal manifestations were present at the time of purpura in 18% of patients and afterwards in 13.1%. Studies have found that renal involvement follows the purpuric rash in 20-25% of cases, in the first 3 months.1,6 The most frequent alterations were proteinuria and microhematuria, as has been described,1,6,25 with nephrotic proteinuria, acute renal injury and hypertension happening occasionally, as was the case.6,25
Some of the factors associated with a possible poor prognosis include age older than 8 years, abdominal complaints, persistent purpura and a higher degree of histological renal involvement.5,26,27 In this study, we found no association between age and a poorer outcome, but found a correlation between abdominal and renal involvement.
There was no possibility to compare histological degree and prognosis, as there were no kidney biopsies performed. No correlation was found between sex and poorer outcomes. There was a negative correlation between joint involvement and recurring episodes, showing that it may be a possible factor associated with a better prognosis.
HSP treatment depends on the severity of the case. It is mostly symptomatic when there is no renal involvement, with steroids used in some more severe cases, particularly when the kidney is affected.1,2,5,6 A Cochrane review showed no prevention of renal disease with the use of steroids at follow-up after 1, 3, 6 and 12 months28,29, as did another long-term study with a follow-up of 7.7 years.27,30
Even so, although not altering the natural history of the disease, preventing symptoms or recurrence10,20, they are effective as treatment, with a systematic review and several case control studies demonstrating a reduction in severity and duration of abdominal and articular pain, and the resolution of hematuria and proteinuria.17,25,27 The most effective treatment for established nephritis is not consensual.5
So, as the majority of non-severe cases has spontaneous recovery, steroids cannot be recommended for all patients with HSP, but they can be used in more severe cases.6 In our analysis, about one fifth of the children was treated with steroids. Consistent with what has been described, after analyzing the data, no protective effect on the outcome was demonstrated for corticotherapy, with no correlation having been found between the use of steroids and renal involvement, and it being indeed correlated with hospitalization, recurrence and persistence of manifestation. This correlation can be explained by the fact that the patients having indication for and receiving corticotherapy were inherently more severe and so, more prone to poorer outcomes, as they had, as previously stated, severe or persistent symptoms or had already developed renal involvement.
The follow-up has been largely recommended in literature to be superior to 6 months5,31, mostly due to renal involvement happening commonly during that period, and our institutions protocol recommends it for more than 1 year, as the possibility of developing or maintaining renal manifestations and recurrence is considered common.
However, the design of this study may have resulted in some limitations that are important to bear in mind. Its retrospective design may have led to the loss of patients with this diagnosis that werent then included in the study. So, it would be important for future research to investigate this pathology in a prospective manner, to avoid selection bias.
In conclusion, HSP is a common and mostly benign diagnosis of childhood, although it can have serious and long-term complications, predominantly associated with nephritis, that can arrive at any time during follow-up. So, careful vigilance of this patients is necessary, allied with the security that most cases have spontaneous resolution and need no specific therapy. More studies are necessary in defining clear indications for steroid use and the most effective treatment for established nephritis.
References
1. Hetland LE, Susrud KS, Lindahl KH, Bygum A. Henoch-Schönlein purpura: a literature review, Acta Dermato Venereologica, 2017 Nov 15; 97(10), 1160-1166. [ Links ]
2. Bluman J, Goldman RD. Henoch-Schönlein purpura in children: limited benefit of corticosteroids, Canadian Family Physician, 2014 Nov; 60(11): 1007-1010. [ Links ]
3. Yang YH, Tsai IJ, Chang CJ, Chuang YH, Hsu HY, Chiang BL. The interaction between circulating complement proteins and cutaneous microvascular endothelial cells in the development of childhood Henoch-Schonlein purpura, PLoS One 2015; 10: e0120411. [ Links ]
4. He X, Yu C, Zhao P, Ding Y, Liang X, Zhao Y, et al. The genetics of Henoch Schonlein purpura: a systematic review and meta-analysis, Rheumatology International 2013; 33: 1387-1395. [ Links ]
5. McCarthy HJ1, Tizard EJ. Clinical practice: diagnosis and management of Henoch-Schönlein purpura, European Journal of Pediatrics, 2010 Jun; 169(6): 643-650. [ Links ]
6. Trnka P. Henoch-Schönlein purpura in children, Journal of Paediatrics and Child Health, 2013 Dec; 49(12): 995-1003. [ Links ]
7. Chen O, Zhu XB, Ren P, Wang YB, Sun RP, Wei DE. Henoch-Schonlein purpura in children: clinical analysis of 120 cases, African Health Sciences 2013; 13: 94-99. [ Links ]
8. Ozen S, Pistorio A, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria, Annals of the Rheumatic Diseases, 2010 May; 69(5): 798-806. [ Links ]
9. Narchi H. Risk of long term renal impairment and duration of follow up recommended for Henoch-Schonlein purpura with normal or minimal urinary findings: a systematic review. Arch Dis Child. 2005 Sep; 90(9): 916-920. [ Links ]
10. Saulsbury FT. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature, Medicine (Baltimore), 1999 Nov; 78(6): 395-409. [ Links ]
11. Weiss PF, Feinstein JA, et al. Effects of corticosteroid on Henoch-Schönlein purpura: a systematic review, Pediatrics, 2007 Nov; 120(5): 1079-1087. [ Links ]
12. Jauhola O, Ronkainen J, et al. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: a 6-month prospective study, Archives of Disease in Childhood, 2010 Nov; 95(11): 871-876. [ Links ]
13. Rostoker G. Schönlein-Henoch purpura in children and adults: diagnosis, pathophysiology and management, BioDrugs 2001;15(2):99-138. [ Links ]
14. Sohagia AB, Gunturu SG, Tong TR, Hertan HI. Henochschonlein purpura – a case report and review of the literature, Gastroenterol Res Pract 2010; 2010: 597648. [ Links ]
15. Weiss PF, Klink AJ, Luan X, Feudtner C. Temporal association of Streptococcus, Staphylococcus, and parainfluenza pediatric hospitalizations and hospitalized cases of Henoch-Schönlein purpura, J Rheumatol. 2010; 37: 2587-2594. [ Links ]
16. Trapani S, Micheli A, et al. Henoch-Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature, Semin Arthritis Rheum. 2005 Dec; 35(3): 143-153. [ Links ]
17. Jauhola O, Ronkainen J, et al. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: a 6-month prospective study, Arch Dis Child. 2010 Nov; 95(11): 871-876. [ Links ]
18. Ting TV. Diagnosis and management of cutaneous vasculitis in children, Pediatr Clin North Am. 2014 Apr; 61(2): 321-346. [ Links ]
19. Dudley J, Smith G, et al. Randomised, double-blind, placebo-controlled trial to determine whether steroids reduce the incidence and severity of nephropathy in Henoch-Schonlein Purpura (HSP), Arch Dis Child. 2013 Oct; 98(10): 756-763. [ Links ]
20. Peru H, Soylemezoglu O, et al. Henoch-Schonlein purpura in childhood: clinical analysis of 254 cases over a 3-year period, Clin Rheumatol. 2008 Sep; 27(9): 1087-1092. [ Links ]
21. Ha TS, Lee JS. Scrotal involvement in childhood Henoch-Schönlein purpura, Acta Paediatr, 2007 Apr; 96(4): 552-555. [ Links ]
22. Hong J, Yang HR. Laboratory markers indicating gastrointestinal involvement of Henoch-Schönlein purpura in children, Pediatr Gastroenterol Hepatol Nutr. 2015 Mar; 18(1): 39-47. [ Links ]
23. Kim S, Yoon J, Jeong S. Comparison of the clinical manifestations and prognosis of Henoch-Schonlein purpura in children with and without abdominal pain. Korean J Pediatr Gastroenterol Nutr. 2011; 14: 359-367. [ Links ]
24. Ozen S, Ruperto N, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides, Ann Rheum Dis. 2006 Jul; 65(7):936-941. [ Links ]
25. Chen J-Y, Mao J-H. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management, World J Pediatr 2015; 11: 29-34. [ Links ]
26. Chang WL, Yang YH, Wang LC, et al. Renal manifestations in Henoch–Schönlein purpura: a 10-year clinical study, Pediatr Nephrol. 2005; 20(9):1269-1272. [ Links ]
27. Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch–Schönlein purpura: a randomized, double-blind, placebo-controlled trial, J Pediatr. 2006; 149(2): 241-247. [ Links ]
28. Chartapisak W, Opastirakul S, Hodson EM, Willis NS, Craig JC. Interventions for preventing and treating kidney disease in Henoch-Schönlein purpura (HSP), Cochrane Database Syst Rev 2009; (3): CD005128. [ Links ]
29. Hahn D, Hodson EM, Willis NS, Craig JC. Interventions for preventing and treating kidney disease in Henoch-Schönlein Purpura (HSP), Cochrane Database Syst Rev. 2015 Aug 7; (8): CD005128. [ Links ]
30. Jauhola O, Ronkainen J, Koskimies O, Ala-Houhala M, Arikoski P, Hölttä T, et al. Outcome of Henoch-Schönlein purpura 8 years after treatment with a placebo or prednisone at disease onset, Pediatr Nephrol. 2012; 27(6): 933-939. [ Links ]
31. Narchi H. Risk of long term renal impairment and duration of follow up recommended for Henoch–Schönlein purpura with normal or minimal urinary findings: a systematic review, Arch Dis Child. 2005; 90(9): 916-920. [ Links ]
Maria João Gaia, MD
Department of Pediatrics / Neonatology, Centro Hospitalar de Vila Nova de Gaia/Espinho; Rua Francisco Sá Carneiro, s/n | 4400-129 Vila Nova de Gaia
E-mail: maria.stgaia@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: Oct 29, 2019
Accepted in revised form: Dec 18, 2019














