Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.34 no.4 Lisboa dez. 2020
https://doi.org/10.32932/pjnh.2021.01.103
REVIEW ARTICLE
Extracorporeal light chains removal – What role does this play in 2020?
Inês Coelho1, Hugo Ferreira2, Teresa Chuva2, Ana Paiva2, José Maximino2
1 Hospital Amato Lusitano, Unidade Local de Saúde de Castelo Branco, EPE, Castelo Branco, Castelo Branco, Portugal.2 Instituto Português de Oncologia do Porto Francisco Gentil EPE, Porto, Porto, Portugal.
ABSTRACT
Multiple Myeloma (MM) is characterized by a neoplastic proliferation of plasma cell clones producing monoclonal immunoglobulin. Manifestations of the disease are heterogenous and include dialysis‑requiring acute kidney injury (AKI) caused mainly by cast nephropathy (CN). It is known that early and rapid decrease in serum free light chains (sFLC) levels is particularly important for renal recovery, which has led to a renewed interest in extracorporeal methods of removal of sFLC.
In this review we will discuss the management of light chain CN focusing on extracorporeal light chains removal modalities and their indication.
Keywords:Acute kidney injury, cast nephropathy, dialysis, multiple myeloma, serum free light chains
INTRODUCTION
Multiple Myeloma (MM) is characterized by a neoplastic proliferation of plasma cell clones producing monoclonal immunoglobulin. The type of monoclonal immunoglobulin could be a heavy chain plus a light chain (LC), or more frequently, just an excess of LCs, which can be nephrotoxic. MM is an heterogenous disease with different clinical manifestations, the most important being those that form the acronym CRAB: hyper Calcemia, Renal Failure, Anemia, Bone lesions1. Variable cytogenetics affects disease evolution, treatment response and prognosis2. The incidence of renal disease is undetermined, but up to 50% of patients may have renal involvement during the course of the disease3. The most serious complication is dialysis‑requiring acute kidney injury (AKI) (1–13%)4, which worsens the prognosis of the disease and is caused in the majority of the cases by myeloma cast nephropathy (CN)1.
Although the frequency of renal disease in MM has not changed for several years, both hematologic response and overall survival for patients with severe AKI has significantly improved5. MM was treated for many years with different medical protocols and the improvement in outcomes was achieved by the introduction of highly active chemotherapeutic agents. The mainstay of renal recovery is recognized as an early and rapid decrease in serum free light chains (sFLC) levels, which has led to a renewed interest in extracorporeal methods of removal of sFLC, as an adjuvant of medical therapy6.
In this review we will discuss the management of light chain CN, focusing on extracorporeal light chains removal modalities and their indication.
MECHANISMS OF KIDNEY INJURY BY FREE LIGHT CHAINS AND ITS ASSESSMENT
sFLC circulate as monomers (predominantly κ ≅ 25 kDa) and dimers (predominantly λ ≅ 50 kDa) and their levels are influenced by synthesis, volume of distribution, and elimination. The concentration of k and λ light chains is similar in the different body compartments (serum, extravascular compartment and interstitial tissue fluid)7, with only 15‑25% of sFLC being in the intravascular compartment. Under normal biologic conditions, sFLC have a high synthetic rate, reflected in their half‑life of only 2–4h for k sFLC and 3–6 h for λ sFLC. Half‑life can increase up to 3 days in patients with no renal function8.
In MM, massive production of sFLC overwhelms the absorptive capacity of the proximal tubule, leading to intratubular obstruction of the distal tubules9. Recent evidence has shown the importance of the interaction between FLCs and Tamm‑Horsfall protein (THP) in the development of CN. Immunoglobulin free light chains are toxic to the tubules when coprecipitation occurs with TPH, resulting in the formation of obstructing tubular casts in the distal tubules, particularly if the patient is volume depleted10, as well as direct proximal tubular injury through the nuclear factor (NF) κB pathway9.
Patients presenting with CN have usually been exposed to precipitating factors, such as dehydration, infection, non‑steroidal anti‑inflammatory agents (NSAIDs), nephrotoxic antibiotics and radiocontrast agents11 that exert either a direct tubule‑interstitial toxicity or increase the concentration of sFLC in the distal nephron, enhancing their precipitation. In such circumstances, the application of general measures of treatment are crucial and are described in the next section.
The ability to measure sFLC levels routinely and reliably was a hallmark in the assessment and treatment of MM. CN is usually associated with sFLC levels >500–1000 mg/L. In the presence of sFLC <500 mg/L, when there is no renal biopsy documentation of CN, diagnosis of CN should be carefully reconsidered, and chemotherapy alone should be sufficient to obtain a rapid reduction of sFLC12. On the other hand, concentrations of sFLC over 1500mg/dL and Bence‑Jones proteinuria are almost certainly associated with CN, obviating renal biopsy13. Light chain CN should be treated without delay to allow renal recovery.
TREATMENT OF AKI DUE TO CAST NEPHROPATHY
For patients with MM who have a confirmed or suspected diagnosis of light chain CN, the treatment is divided into 3 steps14:
General measures:
– Eviction of nephrotoxic agents, such as NSAIDs and radiocontrast agents;
– Avoidance of RAAS inhibitors: angiotensin‑converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs);
– Intensive intravenous or oral fluid therapy maintaining high urine output (at least 3L/day);
– Treatment of hyperuricemia;
– Correction of hypercalcemia;
Specific Treatment:
– Early directed therapy, followed by Intensive chemotherapy with hematopoietic stem‑cell transplantation (HSCT) rescue, in selected patients;
Extracorporeal removal of light chains.
Proteasome inhibitor‑based chemotherapy with high‑dose dexamethasone is the first line therapy for most patients (such as bortezomib, cyclophosphamide, and dexamethasone, or CyBorD) that will target the light‑chain production and reduce the concentration of pathogenic free light chains15,16. With these therapeutic regimens, the reduction of serum free light chain concentrations is more rapid, and kidney recovery is significantly improved15.
Recent studies have shown that HSCT may be safe and effective in patients with renal failure17, changing the idea that renal failure is always exclusion criteria for HSCT. The Mayo Clinic reported a 10‑year retrospective review of 30 patients receiving autologous HSCTs for MM with serum creatinine >3 mg/dl (50% required dialysis), in which hematologic response was achieved in all patients on dialysis, although only one of 15 was able to discontinue dialysis, and in patients not dialysis dependent there was an improvement in glomerular filtration rate (GFR) from 15 to 19.4 mL/min/1.73 m2 18.
The goal for fluid management is a daily urine output of approximately 3 liters. The patient should be euvolemic, and loop diuretics should be avoided as much as possible, because they decrease THP solubility by increasing intraluminal sodium, promoting cast formation12.
Hypercalcemia should be corrected to prevent renal vasoconstriction and volume depletion from nephrogenic diabetes insipidus. Bisphosphonate therapy (zoledronate and pamidronate) should be adjusted accordingly to renal function and careful monitoring of any side effects is necessary19.
Allopurinol can be used to treat hyperuricemia, which will reduce urate formation by inhibiting xanthine oxidase activity; another option is rasburicase, which rapidly lowers uric acid. However, MM has a low risk of tumor lysis syndrome, and patients seldom need rasburicase12.
INDICATIONS FOR EXTRACORPOREAL LIGHT CHAINS REMOVAL
The relatively small molecular weight of light chains allows their removal by techniques of extracorporeal depuration.
The rationale for extracorporeal light chains removal is based upon a possible reduction in dialysis dependency among survivors, additionally lowering sFLC concentration. However, the use of extracorporeal methods in the treatment of light chain CN remains controversial, and some clinicians do not use these therapies in this setting. Extracorporeal light chains removal must always be used with the early institution of specific treatment to reduce light chain production, by targeting the cell clone, most commonly with bortezomib‑based regimens.
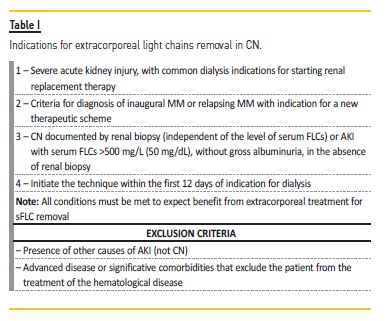
The protocol of our institution12 is presented in Table I and was based on the protocol of Onconephrology Work Group of the Italian Society of Nephrology. Delayed AKI diagnosis (>1 month), unstable cardiovascular condition and eligibility only for slow‑acting chemotherapies require careful evaluation to weigh the benefits and risks and define treatment indication12.
MODALITIES OF EXTRACORPOREAL LIGHT CHAINS REMOVAL
Three light chain removal strategies have been used in CN:
Therapeutic plasma exchange
The application of extracorporeal therapy for CN initially began with the use of therapeutic plasma exchange (TPE), but its extracorporeal removal of sFLC was disappointing. The largest randomized controlled trial of plasma exchange, which included 104 patients with severe AKI associated with MM, failed to demonstrate any benefit of plasma exchange for either renal recovery or overall patient survival20.
However, this trial has been criticized, because the sample size was small and there was no biopsy confirmation of CN21.
Hutchison et al.22 demonstrated that TPE increased removal rates of sFLC by approximately 25% but concentrations were not reduced below toxic levels (500 mg/L) at 4 weeks. The lack of success of plasma exchange may be explained by the limited duration and frequency of this procedure, combined with on‑going high synthetic rate and re‑entry of FLCs from extravascular compartments23. Another important limitation regarding plasmapheresis is that the majority of available studies were designed before the advent of proteasome inhibitor bortezomib, used in the current regimens of MM treatment, so translating these studies’ results into current clinical practice is not advisable12.
In 2010, an IMWG consensus statement acknowledged that “The role of plasma exchange in patients with suspected light chain CN and renal impairment is controversial”24. More recently, Premuzic et al.25 compared sFLC concentrations in MM patients treated with chemotherapy alone (bortezomib) or in combination with plasma exchange (2–5 sessions). There was no significant difference in outcome between the two groups, but reductions in sFLC concentrations post‑treatment were associated with improved survival. Hence, the use of TPE in CN cannot be recommended based on current evidence12.
However, cryoglobulinemia and hyperviscosity syndrome as a part of of MM could be indications for TPE.
High‑cutoff hemodialysis
High‑cutoff (HCO) hemodialysis has emerged as a method of extracorporeal removal of sFLC additional to chemotherapy in the treatment of CN. Prolonged dialysis (6‑to 8‑hour sessions) is performed with a hemodialyzer with a large pore size (45–60 kD). In vitro studies showed that HCO hemodialysis could achieve removal of 90% of free light chains over a 3‑week period22.
Two large multicenter, prospective, randomized controlled trials, EuLITE and MYRE, have been undertaken to determine whether HCO hemodialysis improves patients’ outcomes, in the era of bortezomib‑based chemotherapy. It should be noted that there were several differences in the design of these two trials: the protocols differed in pre‑dialysis care, initial chemotherapeutic regimens, intensity of dialysis, and type of dialyzers used21. Here, we summarize the main results of these trials.
1. EuLITE Trial
The European Trial of Free Light Chain Removal by Extended Haemodialysis in Cast Nephropathy (EuLITE)26 compared HCO hemodialysis to standard high‑flux hemodialysis (HF‑HD) in 90 patients with newly diagnosed MM and associated CN, treated with bortezomib‑based chemotherapy. There were 43 patients in the HCO group and 47 patients in the HF‑HD group. The chemotherapy regimen included bortezomib (1 mg/m² on days 1, 4, 8 and 11 of a 21‑day cycle), doxorubicin, and dexamethasone. The treatment protocol was two 1.1 m² filter in series (HCO1100; Gambro); 6‑h session at baseline, then 8‑h sessions on days 2, 3, 5, 6, 7, 9, and 10; from day 12, 8‑h sessions on alternate days, reducing to 6‑h sessions on alternate days from day 21; 60 g albumin was perfused at each session. Following the first full protocol dialysis, a greater reduction in sFLC concentrations was observed for the HCO compared to the high‑flux protocols (κ patients 75.6% vs 20.2%; λ patients: 71.2% vs 9.1%, p<0.001). However, after3 weeks of treatment, there was no difference in the reduction of sFLCs between the two groups, nor in the overall proportion of patients with renal recovery (HCO: 58.1%; high‑flux: 66.0%). Unfortunately, HCO hemodialysis was associated with increased lung infections in the first 3 months (p=0.014) and a reduced overall survival at 2 years (55.8% and 76.6%, respectively). In summary, the EuLITE HCO protocol did not result in an improved outcome compared to standard high‑flux dialysis.
A recent phase 2 multicenter controlled trial from the working group of the EuLITE trial27 showed that HCO hemodialysis did not improve clinical outcomes for patients with de novo MM and CN who required hemodialysis for acute kidney injury and who received a bortezomib‑based chemotherapy regimen relative to those receiving standard high‑flux hemodialysis.
2. MYRE TrialThe MYRE trial28 compared patients with dialysis‑requiring AKI from biopsy‑proven CN receiving bortezomib‑based chemotherapy and either standard dialysis or HCO‑HD.
There were 46 patients in the HCO group and 48 patients in the HF‑HD group. The chemotherapy regimen included bortezomib (1.3 mg/m² on days 1, 4, 8, and 11) and dexamethasone. The protocol of treatment was single membrane 2.1m² dialyser (Theralite; Gambro); 5 h per session; eight sessions for 10 days, and thereafter three sessions per week if needed, until completion of three cycles of chemotherapy (5 h/session); if serum albumin was less than 25 g/L before hemodialysis, 20 g albumin was perfused after dialysis. Considering hematological response, there were statistically significant differences at 3 months (89.1% in the HCO group; 65.2% in the HF‑HD group; p=0.003), but not at 6 months (p=0.06).
The primary end point was the rate of hemodialysis independence at 3 months, and the use of HCO hemodialysis compared with conventional hemodialysis did not result in a statistically significant difference in hemodialysis independence at 3 months (p=0.42), but it was significant at 6 months (p=0.04) and at 12 months (p=0.02). It is possible that the benefit observed at 6 to 12 months might be related to a more rapid early decrease in serum free light chain in the HCO group, and, in addition, it is plausible that the tubular damage required more than 3 months for remodelling and regeneration.
Prolonged HCO hemodialysis is not without risk. It is associated with a need for regular phosphate and albumin supplementation (the MYRE trial) as well as a potential increase in infection risk (the EuLITE). Therefore, HCO hemodialysis must still be considered an unproven adjunct therapy until more robust clinical data are reported, and it is likely unwarranted in nondialysis‑dependent AKI.
ADSORPTION
Different strategies have employed FLCs adsorption or a combination of hemodialysis and adsorption, with good results in the effective removal of FLCs.
1. HFR‑SUPRA®The hemodiafiltration with ultrafiltrate regeneration (HFR‑SUPRA®) technique has 3 stages: hemodiafiltration with separated convection, diffusion and adsorption. Figure 1 shows the HFR‑SUPRA® circuit, FLEXYA® monitor, used in our institution.
It provides plasma depuration without the need for plasma or albumin replacement, and maintains the same effectiveness over time, since an adequate anticoagulation is provided to prevent the filters from clotting. The absence of albumin losses (only 0.015% at the end of the procedure29) as well as the absence of potential loss of other proteins of the immune system is an advantage over HCO hemodialysis protocols30. Esquivias‑Motta et al.31 showed that this technique may improve uremic protein‑bound toxin removal, inflammatory state, endothelial damage and oxidative stress when compared with on‑line hemodiafiltration and high‑flux hemodialysis. The reported FLCs removal rate was 51% (range 38–63)32,33, with more efficient clearance for k sFLC (4.9 to 15.3 ml/min) than for λ sFLC (3.2 to 11.5 ml/min), estimating that k sFLC is twice removed than λ29, possibly due to the high molecular weight of the λ chains and the formation of polymeric aggregates, not subject to convective transport. Pasquali et al.32,34 reported two small studies where patients with dialysis‑dependent renal failure due to biopsy‑proven CN treated with HFR‑SUPRA had a significant reduction of sFLCs and a complete recovery of renal function. More recent studies also performed by Italian groups corroborate that the combination of HFR‑SUPRA treatment with chemotherapy in patients with AKI and MM showed a significative renal functional recovery, with favorable cost/benefit ratio and a simple treatment schedule35,36.
In a recent study, Pendon‑Ruiz de Mier et al.29 showed that this technique is effective as an adjunctive treatment for MM in combination with chemotherapy, allowing renal recovery in 33.3% of patients.
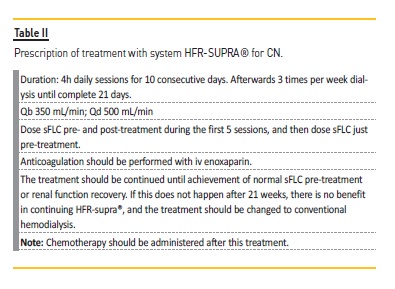
In our institution, we perform the technique according to the protocol12 described in Table II.
2. PMMA‑EAD
Polymethylmethacrylate (PMMA) membranes have adsorption properties. Results have shown that the process using this type of membrane is limited by fast saturation of the membrane adsorption capacity. Dialyser replacement after 2 hours (termed enhanced adsorption dialysis (EAD)) increases the overall adsorption efficiency, particularly for λ FLCs37. Santoro et al.38 reported similar findings using two PMMA membranes in sequence (termed the “DELETE system”).
3. Coupled plasma filtration adsorption
Another technique, which combines a plasma adsorption circuit with a continuous renal replacement therapy, is coupled plasma filtration adsorption (CPFA) and it has been used in the extracorporeal treatment of sepsis and septic shock39. The CPFA circuit consists of a MicropesTM plasmafilter (0.45 m2) in series with a high permeability polyphenylene hemofilter (Kuf 41 mL/h/mm Hg, surface area 1.4 m2).
The plasma flow rate is 30–40 mL/min and the plasma passes into the sorbent adsorption cartridge which contains a 70‑gram styrenic polymer resin. The resin is composed of mesoporous beads; the bead size is 50–100 μm; the average pore diameter is 30 nm, and the surface area is 700 m2 /g = 50,000 m240. In an in vitro study of FLC removal by CPFA using a number of different resins, for patients treated with at least six 4‑hour CPFA sessions using MDR3 resin, sFLC concentrations progressively decreased (p=0.05)41.
Strong evidence supporting the three adsorption techniques described is still lacking. The depurative efficiency could be further improved, increasing surface area of the resin, possibly tailoring more specific resins to FLCs, and the outcomes can be evaluated through studies with a larger number of patients.
CONCLUSION
In this paper, we reviewed the role of sFLC extracorporeal removal during AKI in MM from TPE to more recent techniques. Three major developments have changed the approach to this clinical condition.
Firstly, and more importantly, the availability of highly effective chemotherapeutic drugs which can induce a rapid reduction of tumor burden and sFLC production. According to currently available knowledge, we cannot state that the addition of extracorporeal sFLC removal to standard bortezomib‑based chemotherapy is superior to chemotherapy alone. Second, the ability to routinely and reliably measure sFLC levels, a trustworthy marker of the efficacy of therapy. Third, the improvement in dialyzer technology, providing new devices and membranes that can effectively remove sFLC.
Despite limited data and several controversial aspects, in our opinion it is reasonable that sFLC extracorporeal removal should be used in patients who already need dialysis either for AKI or to control volume and electrolyte disorders. Adsorption techniques such as HFR‑SUPRA provides plasma depuration without the need for plasma or albumin replacement, and maintains the same effectiveness over time, and that is why this is the elected therapy in our institution.
From a conceptual point of view, we can continue to believe in the extracorporeal clearance of sFLC as a form of adjunctive therapy in patients with MM. CN should be promptly recognised without delay before initiation of chemotherapy and effective adjunctive sFLC extracorporeal removal to allow renal recovery.
Only a combination of efforts from many centers will achieve robust results based on high levels of evidence, since clear evidence from randomized controlled trials is still lacking.
References
1. Feehally J, Floege J, Tonelli M, Johnson RJ. Comprehensive Clinical Nephrology. 6th Edition. Elsevier, 2019:767–768.
2. Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015;5(10):e365. [ Links ]
3. Gaballa MR, Laubach JP, Schlossman RL, Redman K, Noonan K, Mitsiades CS, Ghobrial IM, Munshi N, Anderson KC, Richardson PG. Management of myeloma‑associated renal dysfunction in the era of novel therapies. Expert Rev Hematol. 2012;5:51–66.
4. Chanan‑Khan AA, San Miguel JF, Jagannath S, Ludwig H, Dimopoulos MA. Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin Cancer Res. 2012;18:2145–2163.
5. Reule S, Sexton DJ, Solid CA, Chen SC, Foley RN. ESRD due to multiple myeloma in the United States, 2001‑2010. J Am Soc Nephrol. 2016;27:1487–1494.
6. Hutchison CA, Cockwell P, Stringer S, Bradwell A, Cook M, Gertz MA, Dispenzieri A, Winters JL, Kumar S, Rajkumar SV, Kyle RA, Leung N. Early reduction of serum‑free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22:1129–1136.
7. Takagi K, Kin K, Itoh Y, Enomoto H, Kawai T. Human alpha 1‑microglobulin in various body fluids. J Clin Pathol. 1980;33:786–791.
8. Bradwell AR. Serum Free light chain analysis, 4th Edition. The Binding Site Ltd., 2006: 104–107.
9. Hutchison CA, Batuman V, Behrens J, Bridoux F, Sirac C, Dispenzieri A, Herrera GA, Lachmann H, Sanders PW. International Kidney and Monoclonal Gammopathy Research Group: The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2011;8(1):43–51.
10. Sanders PW, Booker BB. Pathobiology of cast nephropathy from human Bence Jones proteins. J Clin Invest. 1992;89(2):630. [ Links ]
11. Walther C, Podoll AS, Finkel KW. Treatment of acute kidney injury with cast nephropathy. Clin Nephrol. 2014;82(1):1–6.
12. Fabbrini P, Finkel K, Gallieni M, Capasso G, Cavo M, Santoro A, Pasquali S. Light chains removal by extracorporeal techniques in acute kidney injury due to multiple myeloma: a position statement of the Onconephrology Work Group of the Italian Society of Nephrology. J Nephrol. 2016;29:735–746.
13. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
14. Finkel KW, Cohen EP, Shirali A, Abudayyeh A. Paraprotein–related kidney disease: evaluation and treatment of myeloma cast nephropathy. CJASN. 2016;11(12)2273–2279.
15. Ludwig H, Adam Z, Hajek R, Greil R, TóthováE, Keil F, Autzinger EM, Thaler J, Gisslinger H, Lang A, Egyed M, Womastek I, Zojer N. Light chain‑induced acute renal failure can be reversed by bortezomib‑doxorubicin‑dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol. 2010;28(30):4635. [ Links ]
16. Sonneveld P, Schmidt‑Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, Zweegman S, Vellenga E, Broyl A, Blau IW, Weisel KC, Wittebol S, Bos GM, Stevens‑Kroef M, Scheid C, Pfreundschuh M, Hose D, Jauch A, van der Velde H, Raymakers R, Schaafsma MR, Kersten MJ, van Marwijk‑Kooy M, Duehrsen U, Lindemann W, Wijermans PW, Lokhorst HM, Goldschmidt HM. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON‑65/GMMG‑HD4 trial. J Clin Oncol. 2012;30(24):2946. [ Links ]
17. Parikh GC, Amjad AI, Saliba RM, Kazmi SM, Khan ZU, Lahoti A, Hosing C, Mendoza F, Qureshi SR, Weber DM, Wang M, Popat U, Alousi AM, Champlin RE, Giralt SA, Qazilbash MH. Autologous hematopoietic stem cell transplantation may reverse renal failure in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15: 812–816.
18. Glavey SV, Gertz MA, Dispenzieri A, Kumar S, Buadi F, Lacy M, Hayman SR, Kapoor P, Dingli D, McCurdy A, Hogan WJ, Gastineau DA, Leung N. Long‑term outcome of patients with multiple [corrected] myeloma‑related advanced renal failure following auto‑SCT. Bone Marrow Transplant. 2013;48:1543–1547.
19. Torregrosa JV, Ramos AM. Uso de bifosfonatos en la enfermedad renal crónica. Nefrologia. 2010;30(3):288–306.
20. Clark WF, Stewart AK, Rock GA, Sternbach M, Sutton DM, Barrett BJ, Heidenheim AP, Garg AX, Churchill DN. Canadian Apheresis Group: Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med. 2005; 143(11):777–784.
21. Finkel KW, and Gallieni M. Extracorporeal removal of light chains new data and continued controversies. Clin J Am Soc Nephrol. 2018;13:1753–1754. https://doi.org/10.2215/CJN.05100418
22. Hutchison CA, Cockwell P, Reid S, Chandler K, Mead GP, Harrison J, Hattersley J, Evans ND, Chappell MJ, Cook M, Goehl H, Storr M, Bradwell AR. Efficient removal of immunoglobulin free light chains by hemodialysis in multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol. 2007;18:886–895.
23. Cserti C, Haspel R, Stowell C, and Dzik W. Light‑chain removal by plasmapheresis in myeloma‑associated renal failure. Transfusion. 2007 Mar;47(3):511–514.
24. Dimopoulos MA, Terpos E, Chanan‑Khan A, Leung N, Ludwig H, Jagannath S, Niesvizky R, Giralt S, Fermand JP, Bladé J, Comenzo RL, Sezer O, Palumbo A, Harousseau JL, Richardson PG, Barlogie B, Anderson KC, Sonneveld P, Tosi P, Cavo M, Rajkumar SV, Durie BG, San Miguel J. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–4984.
25. Premuzic V, Batinic J, Roncevic P, Basic‑Jukic N, Nemet D, Jelakovic B. Role of plasmapheresis in the management of acute kidney injury in patients with multiple myeloma: should we abandon it? Ther Apher Dial. 2018;22:79–86.
26. Hutchison CA, Cook M, Heyne N, Weisel K, Billingham L, Bradwell A, Cockwell P. European trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE): a randomized control trial. Trials. 2008;9:55. [ Links ]
27. Hutchison CA, Cockwell P, Moroz V, Bradwell AR, Fifer L,Gillmore JD, Jesky MD, Storr M, Wessels J, Winearls CG, Weisel K, Heyne N, Cook M. High cutoff versus high‑flux haemodialysis for myeloma cast nephropathy in patients receiving bortezomib‑based chemotherapy (EuLITE): a phase 2 randomised controlled trial. Lancet Haematol. 2019;6(4):e217–e228.
28. Bridoux F, Carron PL, Pegourie B, Alamartine E, Augeul‑Meunier K, Karras A, Joly B, Peraldi MN, Arnulf B, Vigneau C, Lamy T, Wynckel A, Kolb B, Royer B, Rabot N, Benboubker L, Combe C, Jaccard A, Moulin B, Knebelmann B, Chevret S, Fermand JP; MYRE Study Group. Effect of high‑cutoff hemodialysis vs conventional hemodialysis on hemodialysis independence among patients with myeloma cast nephropathy: a randomized clinical trial. JAMA 2017;318(21):2099–2110.
29. Pendon‑Ruiz de Mier M, Ojeda R, Alvarez‑Lara MA, Navas A, Alonso C, Caballero‑Villarraso J, Aljama P, Álvarez MA, Soriano S, Rodríguez M & Martin‑Malo A. Hemodiafiltration with ultrafiltrate regeneration reduces free light chains without albumin loss in multiple myeloma patients. BMC Nephrol. 2020;21:227. [ Links ]
30. Menè P, Giammarioli E, Fofi C, Antolino G, La Verde G, Tafuri A, Punzo G, Festuccia F. Serum free light chains removal by hfr hemodiafiltration in patients with multiple myeloma and acute kidney injury: a case series. Kidney Blood Press Res. 2018;43:1263–1272.
31. Esquivias‑Motta E, Martin‑Malo A, Buendia P, Alvarez‑Lara MA, Soriano S, Crespo R, Carracedo J, Ramírez R, Aljama P. Hemodiafiltration with endogenous reinfusion improved microinflammation and endothelial damage compared with online‑hemodiafiltration: a hypothesis generating study. Artif Organs. 2017;41(1):88–98.
32. Pasquali S, Iannuzzella F, Corradini M, Mattei S, Bovino A, Stefani A, Palladino G, Caiazzo M. A novel option for reducing free light chains in myeloma kidney: supra‑hemodiafiltration with endogenous reinfusion (HFR). J Nephrol. 2015; 28(2):251–254.
33. Pendon‑Ruiz de Mier MV, Alvarez‑Lara MA, Ojeda‑Lopez R, Martin‑Malo A, Carracedo J, Caballero‑Villarraso J, Alonso C, Aljama P. Effectiveness of haemodiafiltration with ultrafiltrate regeneration in the reduction of light chains in multiple myeloma with renal failure. Nefrologia. 2013;33(6):788–796.
34. Pasquali S, Corradini M, Iannuzzella F, Mattei S, Bovino A, Stefani A et al. Extracorporeal free light chains removal in association with chemotherapy in myeloma acute kidney injury: new therapeutic options and old prognostic factors. Online 2013;SA394a. [ Links ]
35. Li Cavoli G, Passanante S, Schillaci O, Servillo F, Zagarrigo C, Li Cavoli TV, Palmeri M, Palma B, Rotolo U. Haemodiafiltration with ultrafiltrate regeneration in the removal of free light chains in multiple myeloma and acute kidney injury. Nefrología. 2018;38(3):337–338.
36. Daidola G, Guarena C, Brustia M, Leonardi G, Vigotti FN, Marciello A, Bianco S, Chiarinotti D, Saltarelli M, Besso L, Biancone L. Efficacy of SUPRA HFR in the treatment of acute renal damage during multiple myeloma. G Ital Nefrol. 2018;35(6). pii: 2018‑vol6. [ Links ]
37. Fabbrini P, Sirtori S, Casiraghi E, Pieruzzi F, Genovesi S, Corti D, Brivio R, Gregorini G, Como G, Carati ML, Viganò MR, Stella A. Polymethylmethacrylate membrane and serum free light chain removal: enhancing adsorption properties. Blood Purif. 2013;35(2):52–58.
38. Santoro A, Grazia M, Mancini E. The double polymethylmethacrylate filter (DELETE system) in the removal of light chains in chronic dialysis patients with multiple myeloma. Blood Purif. 2013; 35(2):5–13.
39. Tetta C, Cavaillon JM, Schulze M, Ronco C, Ghezzi PM, Camussi G, Serra AM, Curti F, Lonnemann G. Removal of cytokines and activated complement components in an experimental model of continuous plasma filtration coupled with sorbent adsorption. Nephrol Dial Transplant. 1998;13:1458–1464.
40. La Manna G. and Donati G. Coupled plasma filtration adsorption: a multipurpose extracorporeal detoxification therapy. Blood Purif. 2018;46:228–238.
41. Mancini E, Sestigiani E, Gissara Z, Palladino G, Santoro A (2011) Light chain removal by means of adsorption in the extracorpeal treatment of myeloma‑induced cast nephropathy. XLVIII ERAEDTA Congress, Prague, Czech (abstract available at http://www.abstracts2view.com/era_archive/view.php?nu=ERA11L_1648) [ Links ]
Inês Dionísio Coelho, MD
Hospital Amato Lusitano, Unidade Local de Saúde de Castelo Branco, EPE
Av. Pedro Alvares Cabral, 6000‑085
Castelo Branco
Email: dcoelho.ines@gmail.com
Disclosure of potential conflicts of interest:none declared
Received for publication: Aug 31, 2020
Accepted in revised form: Nov 26, 2020














