INTRODUCTION
Sarcopenia is defined by the reduction of mass, strength, and muscle function. This chronic condition, associated with the physiological aging process, is common in patients with chronic kidney disease (CKD) from the early stages of the disease and the more severe the loss of renal function, the greater the risk of sarcopenia.1,2,3
The importance of maintaining muscle mass and physical and metabolic functions in the elderly is well‑recognized.
Progressive sarcopenia is ultimately central to the development of frailty, na increased likelihood of falls, and impairment of the ability to perform daily living activities. These alterations in muscle also play an importante role in many diseases, such as CKD.4
Indeed, sarcopenia negatively affects skeletal muscle health in CKD, impairing the quality of life of these patients and contributing to morbidity and mortality.5 Therefore, early diagnosis and treatment of sarcopenia are crucial in all CKD patients to prevent the adverse outcomes related to this syndrome. These evaluations should be incorporated into pre‑dialysis, peritoneal dialysis (PD), hemodialysis (HD), and kidney transplant patients.6
This article aims to review the impact of CKD on sarcopenia. We summarize general definitions of sarcopenia, its pathophysiology, clinical implications, as well as a diagnostic and therapeutic approach.
DEFINITION AND PATHOPHYSIOLOGY OF SARCOPENIA
The diagnosis of sarcopenia, developed by the European Working Group on Sarcopenia in Older People (EWGSOP), requires documentation of both low muscle mass and low muscle function (strength or performance).7 Pre‑sarcopenia is defined as loss of skeletal muscle mass alone.8
There is another terminology for skeletal muscle loss conditions that must be differentiated from sarcopenia, including dynapenia, protein‑energy wasting (PEW), frailty, and cachexia. Table I displays the definitions of these potentially confusing terms. This is particularly important for a practical approach as, for example, a patient who is
diagnosed with sarcopenia will benefit from physical therapy to improve muscle mass and function, whereas a patient with PEW or cachexia will benefit from greater involvement of nutrition to combat the significant loss of muscle mass.9
Sarcopenia can be considered “primary” (or age‑related) when no other cause is evident or “secondary” when causes are present, concomitante or not with aging. In the last category, besides activity‑related sarcopenia (sedentary lifestyle) and nutrition‑related sarcopenia (inadequate dietary intake of energy and/or protein, malabsorption conditions), the group of disease‑related sarcopenia, such as CKD, is included.7
CKD - chronic kidney disease, ESRD - end‑stage renal disease
With the increase in the world’s older population, sarcopenia is becoming a major clinical problem in public health, and based on the results of a meta‑analysis, the overall prevalence of sarcopenia is 10%.10
Several mechanisms are involved in the pathophysiology of sarcopenia, including age‑related factors (mitochondrial dysfunction, apoptosis, atherosclerosis), hormonal imbalance (declines in serum testosterone and growth hormone (GH)), insulin resistance, immobility, neuromuscular integrity, changes in circulating pro‑inflammatory
cytokines, and muscle fat content.7 With aging, more phenomena lead to the development of sarcopenia. There is a progressive reduction in physical activity, increased body fatness, inflammation (increased cytokine levels), insulin resistance,
nutritional deficiencies (protein, vitamin D, and other micronutrients), and reduced muscle protein synthesis.11
At the molecular level, sarcopenia may be a result of a disproportionate decrease in skeletal muscle protein synthesis and/or na increased protein breakdown. Proteolytic and lysosomal‑dependent degradation, manipulated by myokines (such as myostatin and IL‑6) may explain this relationship. Myostatin affects muscle wasting by disrupting skeletal muscle satellite cells and an up‑regulation of E3 ligases instigating protein breakdown by the proteasome. IL‑6 signaling, present in chronic systemic inflammation, has been associated with myogenesis. In CKD, these myokines are elevated, from the early stages.5
Furthermore, elevated levels of angiotensin II stimulate caspase‑3, which enhances actin cleavage, allowing actin and myosin to be degraded by the ubiquitin‑proteasome
system. Angiotensin II is also responsible for low circulating and skeletal IGF‑1
levels, which enhances muscle wasting as well. This may explain the association of angiotensin converting enzyme inhibitors with increased muscle mass.11,12In CKD, it has been also observed that FGF23 and Klotho induced muscle atrophy via reduction of insulin/IGF‑I signaling.13
Also, there are understood factors directly related to muscle mass, strength, and function. Future studies may help to promote some beneficial interventions, including exercise and diet.
METHODS TO EVALUATE SARCOPENIA
Although sarcopenia has been emphasized as leading to dynapenia (muscle weakness), a more appropriate focus in sarcopenia includes the central function of muscle. As such, mobility is perhaps the best way to evaluate muscle impairments and serves as a primary indicator of functional ability.14
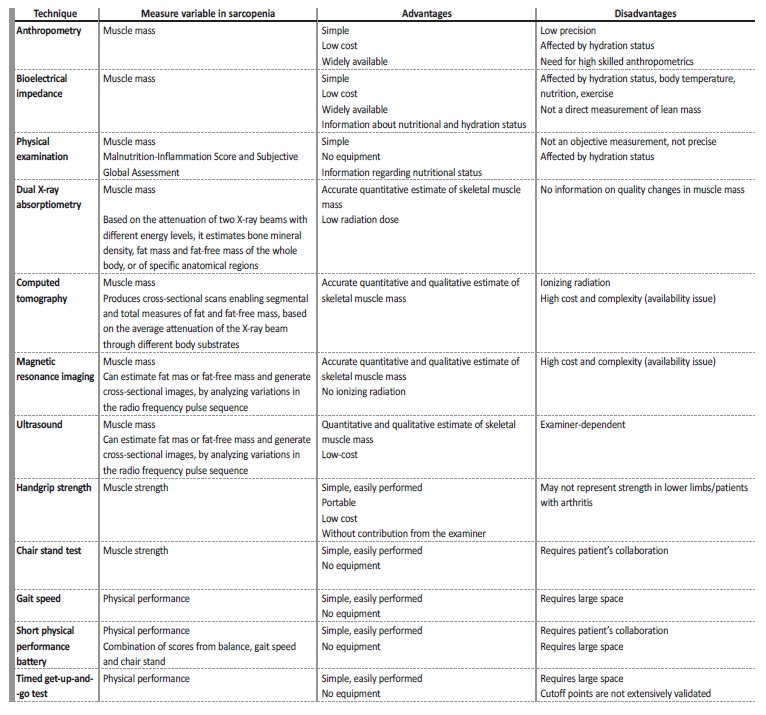
The measurable variables to evaluate sarcopenia are muscle mass, muscle strength, and physical performance, as illustrated in Table II.
For clinical use, handgrip strength is a good simple measure of muscle strength and the short physical performance battery, including the usual gait speed, and the timed get‑up‑and‑go test, can serve as performance measurements.7
Regarding muscle mass, there are some imaging methods for muscle mass assessment available. Computed tomography (CT) and magnetic resonance imaging (MRI) are considered gold standards to measure skeletal muscle mass in research. However, although these modalities allow precise evaluation of muscle mass, they are of limited availability, costly, time‑consuming, and complex technologies. Dual X‑ray absorptiometry (DXA) is the preferred alternative method for clinical use and currently the method used the most often to detect sarcopenia, as it requires less time and only a low radiation dose. In this method, the appendicular skeletal muscle mass (ASMM) analyses the sum of the muscle mass of the arms and legs and there is na ASMM index (the ratio between ASMM and height squared) cut‑off for diagnosing sarcopenia in men and women.8
Bioimpedance analysis (BIA) estimates the volume of fat and lean body mass and it has also been proposed as a practical alternative to imaging modalities in multiple clinical settings.15 Equations to estimate skeletal muscle index by BIA have been proposed by the EWGSOP and are widely accepted screening tools for low muscle mass in the elderly population.7
SARCOPENIA IN CHRONIC KIDNEY DISEASE
Prevalence, risk factors and comorbidity
There is a greater prevalence of secondary sarcopenia due to CKD than among subjects with normal renal function. The decline in muscle contributes to physical inactivity and multiple outcomes, even during the early stages of CKD.2,17,18,19,20
Table III summarizes the conclusions of some studies that have analyzed the prevalence of sarcopenia in CKD patients. The different findings suggest that there is not yet na agreement on which operational criteria to apply when diagnosing sarcopenia in CKD.
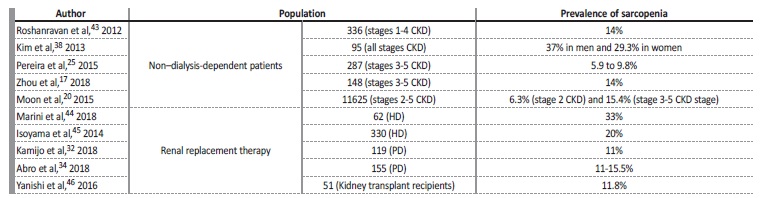
Table III Prevalence of muscle loss conditions in chronic kidney disease.17,20,25,32,34,38,43,44,45,46
CKD - chronic kidney disease, HD - hemodialysis; PD - peritoneal dialysis; ESRD - end‑stage renal disease.
Skeletal muscle is considered the main source of protein in the body and protein is essential for antibody production, wound healing, and white blood cell production during acute or chronic illnesses.
With the development of sarcopenia, the risk of disability and functional impairment is enhanced. It has been observed that HD patients with low lean mass and overhydration have significantly higher mortality.21 Furthermore, a study has proved that in patients
with type 2 diabetes, the low muscle mass is independently associated with the increment of albuminuria, a surrogate marker of adverse renal and cardiovascular outcomes.22 In fact, the sarcopenia‑related‑CKD predisposes a patient to an increased risk of cardiovascular events.23
Multiple studies have already shown that nutritional disturbances are common in patients with CKD, with a predominance of sarcopenia and central obesity.24‑28
In patients with CKD and obesity, the production of inflammatory mediators by adipose tissue also increase the prevalence of cardiovascular events.2
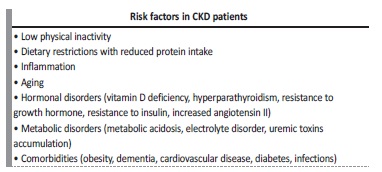
CKD patients manifest a phenotype of accelerated aging as a consequence of the several related risk factors (Table IV). Moreover, this is a ‘chicken‑or-egg’ conundrum, as it is unknown whether reduced physical activity causes muscle loss or loss of muscle causes reduced activity. The loss of skeletal muscle may predispose to a more sedentary lifestyle, increased risk of falls and frailty with subsequently more fractures, hospitalizations, and poorer quality of life.29Indeed, ameliorating physical function will improve the overall quality of life across the spectrum of CKD and this can be achieved by the implementation of exercise.5 Regular muscle mass assessment is strongly recommended in patients with CKD, regardless of the disease stage or the modality of renal replacement therapy.24
Muscle weakness in PD patients is frequent30 and associated with high inflammatory and nutritional markers.31 The associated poor outcomes,30 such as physical disability, decreased quality of life, increased morbidity, and a high mortality rate have also been demonstrated in several studies.32,33
Sarcopenia is even more common in HD patients.34 This subgroup of patients is more vulnerable to multiple metabolic and nutrition derangements, leading to changes in body composition, which can affect handgrip strength and muscle mass measurements.35,36
Published data showed that sarcopenia is a relatively common condition among patients undergoing maintenance HD and is associated with a worse survival rate.37,38
Kidney transplant recipients frequently also exhibit an abnormal body composition similar to sarcopenic obesity.39 Indeed, the impact of sarcopenia in transplant recipients is relevant.40Patients who are sedentary, protein malnourished, and debilitated have been shown to experience increased mortality after many procedures, including vascular, oncologic, and transplant surgeries.41
To date, there are a limited number of studies that have examined the relationship between sarcopenia and decreased kidney function.20,25,26In a systematic review and meta‑analysis, sarcopenia was significantly associated with urinary protein level and decreased renal function, in patients with diabetes, although these analyses were performed using few studies.42
Pathophysiology
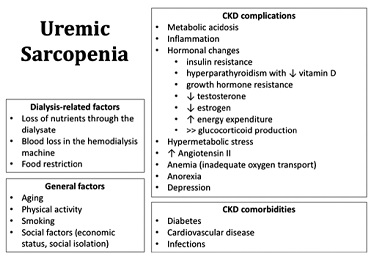
The pathogenesis of uremic muscle loss is complex and multifactorial and is still not fully understood.3,12,43Several mechanisms are summarized in Figure 1, including the conditions of kidney disease itself, dialysis procedure, and typical chronic inflammation present in CKD. These conditions together lead to the dysregulation of the protein synthesis and catabolism, leading to a negative protein balance.16,47
CKD - chronic kidney disease.
Muscle wasting is characterized by increased muscle turnover rate and atrophy of type II muscle fibers.48 The final result is sarcopenia that progresses as CKD worsens.29
The kidney’s non‑inflammatory factors include hormonal imbalances, such as insulin resistance, decreased sex hormones levels, GH resistance, and vitamin D deficiency, and metabolic disorders such as metabolic acidosis.3
Impaired insulin signaling, involving the impact of low vitamin D status, may be responsible for muscle proteolysis.49,50 Insulin is the main hormone responsible for muscle protein synthesis, and vitamin D deficiency not only reduces pancreatic insulin secretion but also diminishes the stimulus for protein synthesis (by decreasing vitamin D receptors present in muscle and reducing the calcium influx from cellular membranes).51 It is now evidenced that patients with sarcopenia and CKD may share insulin resistance and low vitamin D levels.20,48,50
Another phenomenon involved is the metabolic acidosis in CKD. Accumulation of acid in interstitial compartments, even before a fall in serum bicarbonate, can induce muscle damage and inflammation, produce or worsen bone disease, contribute to the progression of CKD and the development of hypoalbuminemia, increasing mortality.22,48
Other hormonal imbalances (low testosterone and GH resistance) are considered a potential cause of increased protein catabolism, by altering IGF‑1 signaling.3
Dialysis‑related factors include the substantial loss of amino acids during the HD procedure and the reduced energy and protein intake.52
Inflammation conditions related to CKD have been demonstrated to be closely associated with sarcopenia,20,38including early systemic markers of atherosclerosis and endothelial dysfunction such as reduction in high‑density lipoprotein levels.18 For instance, fatty acid‑binding protein 4, a novel adipokine primarily synthesized by adipocytes or macrophages that is expressed within human skeletal muscle fibers, has found to be an independent risk factor for sarcopenia in HD patients.53
The renin‑angiotensin‑aldosterone- system is highly activated in CKD and also seems to provide strong input to the development of uremic sarcopenia, as explained earlier.12
Indeed, the chronic inflammation in combination with sarcopenia induces anorexia and fat loss.20
CKD patients have altered cognitive function and mood that could be also associated with sarcopenia. Depression is one of the reasons for reduced appetite and hence for low energy/protein intake.18,38
Other risk factors for sarcopenia among CKD patients, such as drugs, should be investigated. One cross‑section analysis revealed that in patients with pre‑dialysis CKD patients, loop diuretic use was suggested to be a risk factor of sarcopenia.23
Diagnosis
For diagnostic purposes, skeletal muscle mass is the ideal target in the search for muscle abnormalities in CKD. The accuracy of all methods can be affected by CKD‑related
factors, especially hydration status. This is particularly important for methods that cannot distinguish between extracellular and intracellular fluid.54,55
Regarding imaging exams, CT has been studied in CKD patients undergoing HD56,57and pre‑dialysis patients58,59as a measure of quantification of muscle mass and function.
Ultrasonography (US) was also demonstrated to be a valid and reliable method for evaluating the rectus femoris and cross‑sectional area in patients in pre‑dialysis CKD.60,61 This exam represents a valid potential alternative for identifying changes in muscle.8
ASMM, measured by DXA, is currently the most accepted method for muscle mass evaluation in CKD patients.8,55,62
Anthropometry estimates (which include mid‑arm and calf circumference) and BIA are also widely available with low costs and are valid tools in CKD patients, however with lower precision.54
Finally, simplified screening scores for sarcopenia, using age, grip strength, and calf circumference have also been proposed, suggesting a potentially useful tool for estimating the future adverse event risk in patients with CKD.23
Among overweight or obese patients, the best way to assess and normalize skeletal muscle mass remains unclear.63
Table V summarizes some considerations about the diagnostic approach of sarcopenia in CKD patients, from the Kidney Disease Quality Initiative-National Kidney Foundation (KDOQI‑NKF) guidelines.64
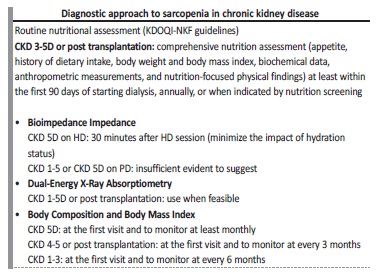
Table V Diagnostic approach of sarcopenia in chronic kidney disease, adapted from Ikizler T et al.64
CKD - chronic kidney disease; HD - hemodialysis; PD - peritoneal dialysis
We think the choice of appropriate measures will depend on cost, clinical experience and the ease of each one. Beyond anthropometry, a physical exam (muscle wasting and palpation) and BIA, an imaging method to evaluate muscle mass may be considered (particularly those which are part of the clinical workup). However, other sarcopenia features may be more easily assessed and therefore applied, such as handgrip strength and gait speed.
There is a need for not only simple techniques capable of monitoring changes in muscle size with disease progression but also appropriate cutoffs for CKD patients. In addition, we should make an effort to apply those available.
Early interventions
Once sarcopenia is identified, interventions should be immediately carried out and monitored by specialized exercise physiologists or other appropriately trained health professionals, and nutritionists.9
Reduced physical activity in sarcopenic subjects, compared to healthy subjects, has been reported, and low muscle mass may reduce ability to participate in physical activities.20 Therefore, physical activity from the early stages of CKD may have protective benefits against the loss of muscle mass.
The KDOQI‑NKF clinical practice guidelines recommend that all dialysis patients should have their physical activity levels assessed and should be regularly encouraged by dialysis staff to increase their physical activity levels.65 Resistance training promotes muscle growth and strength, and theoretically, this may be considered to be the preferred type of exercise to promote physical function in the CKD population.16 Some studies have investigated various modalities of exercise in the CKD population, including patients undergoing HD66 and renal transplant recipients.67
Regarding nutrition, the 2020 updated practice guideline for nutrition in CKD from the KDOQI‑NKF guidelines recommends a protein intake of 0.6‑0‑8 g/kg/day for stages 3‑5
CKD and 1.0‑1.2 g/kg/day for stage 5D CKD, with an energy intake of 30 kcal/kg/day.64 In the case of sarcopenia, it is reasonable to change the dietary scheme to recover the nutritional status.
Interventions to reverse sarcopenia usually include the use of energy and protein supplementation combined with physical exercise.
However, many promising pharmacological interventions for sarcopenia are currently under investigation. In HD patients, angiotensin II receptor blockade has been associated with preserved muscle strength68 and treatment with the androgenic steroid oxymetholone has been associated with an increase in fat‑free mass and handgrip strength.69
Potential therapeutic strategies targeting specific toxins have also been proposed, such as indoxyl sulfate, a uremic toxin that accelerates skeletal muscle atrophy by inducing mitochondrial dysfunction.70,71
Another with promising results is the high‑tone external muscle stimulation that could improve microcirculation and, through this mechanism, reduce kidney damage in elderly patients with CKD and severe muscle atrophy.72
FINAL REMARKS
Sarcopenia represents a devastating complication that not only contributes to a sedentary lifestyle and poor quality of life but also increases the incidence of cardiovascular complications, morbidity, and mortality. CKD is a progressive condition that adversely affects musculoskeletal health and the prevalence of CKD‑sarcopenia
is higher than it is in primary sarcopenia, especially in HD patients.
There is increasing interest in the early diagnosis of sarcopenia, considering the adverse complications of this underestimated condition. In our daily practice, methods and criteria to diagnose sarcopenia in our CKD patients should start to be routinely applied and followed by the implementation of therapeutic strategies that could have improved clinical outcomes.
In the future, better management of sarcopenic patients will be possible by identifying novel therapy pathways, through a better understanding of pathogenic mechanisms, by optimizing the tools to monitor the impact of therapy, and by addressing individual therapeutic approaches.