INTRODUCTION
ANH is the one of the most common findings in prenatal ultrasounds, affecting 1‑5% of all pregnancies.1 The clinical significance of this finding is uncertain, as it represents a wide spectrum of urologic conditions, ranging from insignificant transient physiologic development to significant uropathies such as urinary tract obstruction and vesicoureteral reflux (VUR).2
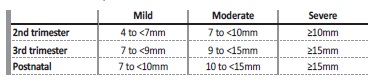
The measurement of the anterior‑posterior diameter of the renal pelvis (APD) in a transverse plane is the most commonly accepted parameter to define hydronephrosis. Even though the cut‑off values for the diagnosis of significant ANH are not consensual, the most widely accepted values are greater than or equal to 4mm in the 2nd trimester and greater than or equal to 7mm in the 3rd trimester.1,2,3,4 5
One of the most accepted and objective grading systems is based on the APD, using different thresholds for the 2nd and 3rd trimesters, classifying ANH in mild, moderate and severe (Table I).1,4,5
Prenatal detection of these urologic anomalies allows early diagnosis of uropathies and intervention to prevent negative consequences, such as urinary tract infection (UTI) or renal failure.2,5,6 However, the majority of the cases of ANH is transient and is not related to significant urologic abnormalities, with this likelihood increasing the lower the grade is.2,6
The clinical challenge is, then, to identify the patients who have significant pathologic abnormalities and who will benefit from early diagnosis and management, and to reduce intervention in those who do not.
The aim of this study was to analyze the clinical and prognostic factors of children with ANH, to establish a cut‑off value and to verify the association between its degrees and negative outcomes, serving as a base for risk stratification that may guide further investigation.
SUBJECTS AND METHODS
A retrospective cohort study was conducted at the author’s institution, a level II hospital, with a hospital catchment area serving 334 081 inhabitants (population of Vila Nova de Gaia and Espinho), 61 225 of them in the pediatric age range.
The sample of this study included the 198 infants born between January 2013 and December 2017 with the diagnosis of antenatal hydronephrosis. ANH was diagnosed by assessment of the anteroposterior diameter (APD) of the renal pelvis, on which stratification was based. We considered the presence of ANH with an APD of the renal pelvis greater than or equal to 4mm in the 2nd trimester and greater than or equal to 7mm in the 3rd trimester.1‑5
ANH was then classified as mild, moderate and severe according to the APD and gestational age (mild if 4 to <7mm in 2nd trimester and 7 to <9mm in the 3rd trimester, moderate if 7 to <10mm in the 2nd trimester and 9 to <15mm in the 3rd trimester, and severe ≥10mm in the 2nd trimester and ≥15mm in the 3rd trimester). Postnatal hydronephrosis was considered if the APD of the renal pelvis was equal to or greater than 7mm, and was classified as mild (7 to <10mm), moderate (10 to <15mm) and severe (≥15mm) (Table I).5,7,8Patients with bilateral dilatation were categorized by the higher grade of APD.
Follow‑up included ultrasounds and complementary exams when indicated. VCUG was performed in cases of bilateral ANH in male patients (early if moderate to severe), in cases of severe hydronephrosis without evidence of obstruction in the MAG3 scan and
considered in persistent moderate hydronephrosis at 6 months and persistent mild hydronephrosis at 12 months (especially if ureteric dilation, duplicated kidney, altered kidney echogenicity or bladder alterations). MAG3 scan was performed in severe cases and considered in persistent moderate hydronephrosis at 6 months and persistent mild hydronephrosis at 12 months. DMSA scan was performed in patients with VUR or with occurrence of UTI. The patients were evaluated for persistence and evolution of hydronephrosis, presence of other urinary tract anomalies, occurrence of UTI and presence of congenital anomalies of the kidney and urinary tract (CAKUT), which we considered to be obstructive disease, renal scarring, altered renal function or structure and vesicoureteral reflux (VUR). The follow‑up period was established until resolution of hydronephrosis or, if persistent, until the end of the study period, with a minimum of 1 year of follow‑up for these patients. The primary outcome of the study was a composite of CAKUT and at least one episode of UTI.
Statistical analysis, including descriptive statistics and inferential statistics, was performed using IBM SPSS Statistics version 23. Categorical variables are presented as absolute or relative frequencies, continuous variables with normal distribution are presented as means and standard deviations and continuous variables without normal distribution are presented as medians and interquartile range. The independent T‑test was used to assess mean differences and the chi‑square test for comparison of proportions. Odds Ratio (OR) and 95% confidence intervals (CI) were used for group risk comparison. Logistic regression was used to identify predictors for the primary outcome, adjusting for confounding variables. All reported p values are two‑tailed, with a value <0.05 indicating statistical significance. The receiver‑operating characteristic (ROC) curve was analyzed to determine the optimal cut‑off for 3rd trimester APD of the renal pelvis to predict negative outcomes (significant uropathy and/or UTI).
RESULTS
The sample included 198 patients with the diagnosis of antenatal hydronephrosis (ANH), and it had a clear male predominance (n=151; 76.3%). The incidence of ANH at our institution was 2.1%. It was bilateral in 89 cases (44.9%).
The diagnosis was made in the second trimester in 37.4% of the cases, with 89.2% of patients having ANH in the third trimester ultrasound.
It resolved antenatally in 12.1% of cases, with a mean value of APD significantly lower in the cases who did (p=0.017). The majority of cases was mild (70.3% in the 2nd trimester and 51.9% in the 3rd trimester), followed by moderate (12.2% in the 2nd trimester and 45.6% in the 3rd trimester) and severe (5.4% in the 2nd trimester and 2.5% in the 3rd trimester). The degree in the 2nd trimester was significantly related to the degree in the 3rd trimester (p<0.001).
The hydronephrosis persisted postnatally in the first month ultrasound in 43.4% of cases, 27.9% of which improved, 58.1% maintained the degree and 14.0% worsened. Overall, of those with persistence of hydronephrosis, 59.3% had a mild degree, 38.4% a moderate degree and 2.3% a severe degree (Table II).
Table II Baseline characteristics of the study sample at diagnosis of antenatal hydronephrosis (ANH) in all subjects and according to the primary composite outcome of the study
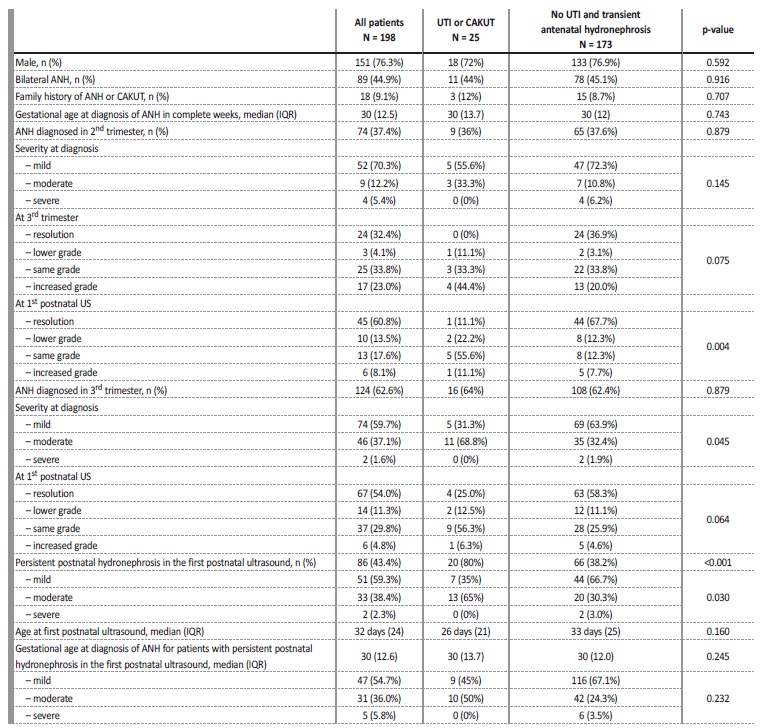
UTI - urinary tract infection; CAKUT - congenital anomalies of the kidney and urinary tract; US - ultrasound
Family history of uropathy was present in 9.1% and of ANH in 6.5%. Diagnostic tests other than ultrasound were necessary in 40 cases (20.2%), with 34 patients undergoing Voiding Cystourethrogram (VCUG) (17.1%), 19 patients MAG3 scan (9.6%) and 16 patients DMSA scan (8.1%). VCUG results were normal in 7 cases (43.8%), showed grade I vesicoureteral reflux (VUR) in 6 cases (17.6%), grade III VUR in 1 case (2.9%) and grade IV VUR in 2 cases (5.9%). The overall VUR rate was 4.5%. MAG3 scan showed non‑obstructive pelvic dilation i 14 cases (73.7%) and obstructive pelvic dilation in 2 cases (10.5%), and 2 cases of altered differential renal function (10.5%). DMSA scan showed altered differential renal function in 5 cases (31.3%), one of them with one kidney failure, kidney scarring in 3 cases (18.8%) and one case of obstructive pelvic dilation (6.3%).
CAKUT (obstructive disease, renal scarring, altered renal function or structure and VUR) was found in 8.6% of patients with ANH (Table III).
Table III Long‑term postnatal outcomes of patients diagnosed with antenatal hydronephrosis*
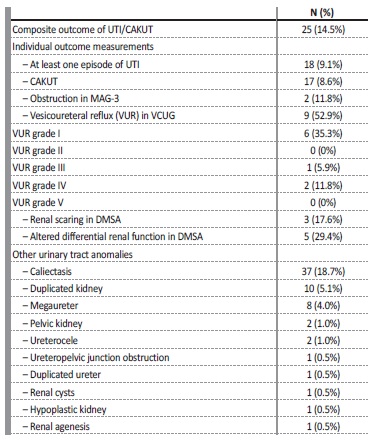
* Age at last follow‑up visit, median in months (IQR): 7.0 (22.3)
UTI - urinary tract infection; CAKUT - congenital anomalies of the kidney and urinary tract; MAG‑3 - Mercaptoacetyltriglycine renogram; VCUG - Voiding Cystourethrogram; DMSA - dimercaptosuccinic acid scan
Other urinary tract anomalies were detected in 24.2% of the patients (n=48), including caliectasis (n=37; 18.7%), duplicated kidney (n=10; 5.1%), megaureter (n=8; 4%), pelvic kidney (n=2; 1%), ureterocele (n=2; 1%), Ureteropelvic junction obstruction (n=1; 0.5%), duplicated ureter (n=1; 0.5%), renal cysts (n=1; 0.5%), hypoplastic kidney (n=1; 0.5%) and renal agenesis (n=1; 0.5%).
One or more episodes of urinary tract infection (UTI) occurred in 9.1% of patients during follow‑up. UTI occurred in 5% of the patients with resolved ANH at the first ultrasound, 9.8% of the mild cases and 20% of the moderate to severe cases (table 3). Antibiotic prophylaxis was started in 33.8% of all patients, 72.3% of which due to moderate to severe degree at birth, 13.8% due to worsening to moderate/severe degree during follow‑up, 10.8% due to occurrence of UTI, and 3.1% due to the presence of VUR.
There was no association between sex and the degree of hydronephrosis or the occurrence of UTI. Postnatal degree was related to the occurrence of UTI (p=0.008). The odd of a patient with moderate to severe hydronephrosis having an UTI is 2.3 times that of a patient with mild hydronephrosis. The occurrence of UTI has no relation to the 2nd or 3rd trimester degree.
The degree in the 3rd trimester was related to postnatal persistence (p<0.001) and postnatal degree (p<0.001), as opposed to the 2nd trimester degree, which showed no correlation. The 3rd trimester and postnatal degree were also associated to the presence of other urinary tract anomalies (p<0.001; p<0.001) and CAKUT (p=0.036; p=0.001).
They had no association with the presence of VUR. The risk of CAKUT was 4.9% for mild cases and 15.8% for moderate to severe cases of ANH (3rd trimester), and 9.8% for mild cases and 25.7% for moderate to severe cases of postnatal hydronephrosis (Table IV).
Table IV Long‑term outcomes by the severity of the hydronephrosis in the first postnatal ultrasound
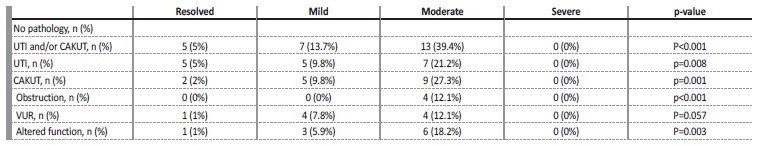
UTI - urinary tract infection; CAKUT - congenital anomalies of the kidney and urinary tract; VUR - Vesicoureteral reflux
The persistence of ANH through the 2nd and 3rd trimester was related to postnatal persistence (p=0.002, OR 3.164), postnatal degree (p=0.007), and the presence of CAKUT excluding VUR (p=0.034). The odd of these patients having significant uropathy excluding VUR was 3.193 times that of the other patients.
The presence of other urinary tract anomalies was also associated with the occurrence of UTI (p<0.001; OR 5.872) and CAKUT (p<0.001; OR 9.341), with the odds of these patients having obstruction, altered function and RVU being 29.5, 5.1 and 6.8 times that of the other patients, respectively. The presence of caliectasis was significantly related to the presence of obstruction (p=0.021).
The presence of VUR was related to the occurrence of UTI (p<0.001; OR 16.923), but not to the sex of the patient.
A regression model was used to identify predictors for the occurrence of the composite outcome of UTI/CAKUT and each of its individual components, adjusting for sex, bilaterality of ANH, gestational age at diagnosis of ANH, severity of ANH at diagnosis, severity of the hydronephrosis in the first postnatal ultrasound and increasing grading at any time. After this analysis, the severity of the hydronephrosis in the first postnatal ultrasound remained significantly associated with all outcomes studied, with p=0.002 for UTI/CAKUT, p=0.049 for UTI and p=0.014 for CAKUT (Table V).
Table V Predictors of UTI/CAKUT among patients diagnosed with antenatal hydronephrosis*
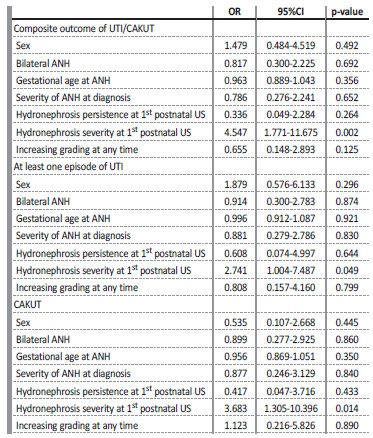
*A regression model was used to predict the composite outcome and its individual componentes adjusting for sex, bilaterality of ANH, gestational age at diagnosis of ANH, severity of the ANH at diagnosis, severity of the hydronephrosis at the first postnatal ultrasound and increasing grading at any time.
ANH - antenatal hydronephrosis, UTI - urinary tract infection, CAKUT - congenital anomalies of the kidney and urinary tract, US - ultrasound
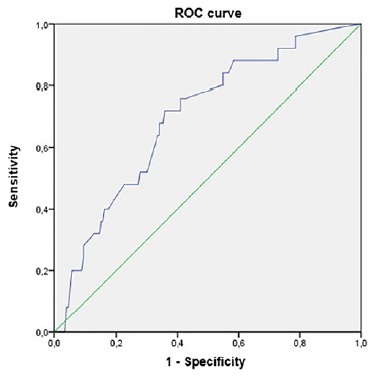
A ROC curve was used to analyze the relationship between the anteroposterior diameter of the renal pelvis in the 3rd trimester and the occurrence of negative outcomes, which we considered to be the presence of CAKUT and/or UTI. The area under the curve was 0.697, and an optimal cut‑off was established at 8.225mm with a sensitivity of 76% and specificity of 59% (Figure 1). We can increase the sensitivity to 88% with a decrease in specificity to 38% using a cut‑off of 7.1mm.
DISCUSSION
ANH is one of the most common prenatal ultrasound findings, with a vast majority of cases being increasingly detected with the widespread availability of these exams. The fact that it represents a sign of a wide range of conditions makes postnatal evaluation and the analysis of these cases essential to define follow‑up.
The reported incidence rates are between 1 and 5% of all pregnancies.1,2,5,9,10 The incidence rate found in our study, of 2.1%, falls into this interval. The majority of our sample were males, agreeing with previous studies,11 although sex was not associated with any negative outcome.
Most cases of ANH are transient and are not associated with clinically significant pathology, with a reported rate in literature between 41‑94%2,5,9,10, consistent with the rate of significant uropathy of 8.6% we described.
The rate of postnatal persistence in our study was 49%, in line with the ones described in literature, between 30‑75%.1,12,13 It usually stabilizes or improves during the postnatal period, though it can worsen in a proportion of cases. A study has reported that about a quarter of the cases shows an aggravation, which is accordant with our findings.11
Most cases of ANH that resolves from the 2nd to the 3rd trimester of pregnancy are not associated with clinically significant pathology, as opposed to the cases in which it persists or worsens, that are much more variable.1 Indeed, we found that the cases of ANH that presente in the 2nd trimester and persists in the 3rd are in fact associated with worse outcomes (higher degree, postnatal persistence and presence of CAKUT excluding VUR), making them a higher risk group.
As reported in previous studies2, the degree of ANH in the 3rd trimester was related to postnatal persistence and postnatal degree, showing it is a reliable parameter to predict postnatal evolution. Na association between the degree of ANH and an increasing incidence of postnatal pathology has also been described in literature.2,4,11,12
We have found a very significant correlation between the degree of ANH in the 3rd trimester, but also at the first ultrasound postnatal, and the occurrence of CAKUT. The risk of any postnatal pathology in a large systematic review was 11.9% for mild, 45.1% for moderate and 88.3% for severe ANH.4 We found smaller numbers in our analysis, though still maintaining a 3‑fold increase in risk from mild to moderate/severe cases, supporting this risk stratification. An exception has been found for VUR, which has shown no correlation with the degree of ANH in several studies2,4, which is consistent with the lack of correlation found in this study.
ANH is more likely to be associated to uropathies if other abnormalities are present, such as caliectasis, parenchymal thinning, ureteric dilation or duplication1,2,14, which we found to be true in our study, since the presence of other urinary tract anomalies was significantly associated with the 3rd trimester and postnatal degrees, the occurrence of UTI and the presence of CAKUT (obstructive disease, renal scarring, altered renal function or structure and VUR). The presence of these additional abnormal findings has been associated with a 12.9 times increased risk of requiring extensive postnatal urologic care15, which is consistent with the 9‑fold increase in risk of uropathy we have found, if these characteristics are present. Thus, these parameters must be taken into account when evaluating the risk of negative outcomes of a patient, in addition to the degree, to define proper follow‑up.
Antenatal hydronephrosis: a five-year retrospective study
Neither gender nor the degree of hydronephrosis (pre and postnatal) were predictive of VUR, which is consistent with the evidence present in literature.16 The overall incidence of VUR in a large systematic review was 8.6%, slightly higher than the 4.5% we found.4 VUR was significantly associated with the occurrence of UTI, with a 17‑fold increase in odds when compared to patients without VUR, as previously described17. These findings further support the need for follow‑up, even in mild ANH cases.
The risk of UTI appears to increase with the degree of hydronephrosis, with a study estimating the incidence of UTI as 7.8% for mild and 20% for moderate/severe hydronephrosis.7,18This is consistente with the rates in our study of 9.8% and 20%, respectively, with the odds of a moderate/severe case having UTI being 2.3 times higher than a mild case. The overall risk of UTI in patients with ANH was 9.1%, in line with previous studies that showed overall rates of 8‑9.4%.17,19 It has been described that UTI develops more frequently in females, which we have not found.19
Through our receiver‑operating characteristic analysis, we found that the optimal cut‑off for 3rd trimester APD of the renal pelvis to predict negative outcomes was 8mm, which is slightly higher than the 7mm commonly accepted. Nevertheless, if a higher sensitivity is intended, the best cut‑off through our analysis is 7mm.
In conclusion, ANH is a common diagnosis that is majorly transitory and not associated with significant pathology, supporting a more conservative approach. Nevertheless, a considerable proportion of cases is associated with negative outcomes, which range from episodes of UTI to altered renal function, and appear to be more likely the more severe the hydronephrosis is. Thus, it is important to establish guidelines to properly guide further investigation, pondering the inherent risks and health care burden associated to ANH, based on risk stratification, for which studies associating degrees of ANH and outcomes are essential.