INTRODUCTION
In December 2019, a cluster of pneumonia cases in Wuhan, China, were linked to a previously unknown coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). This disease was classified as coronavirus disease 2019 (COVID-19), a pandemic as declared by the World Health Organization in March 2020. There are over 150 million reported cases worldwide, approximately 840 thousand in Portugal. COVID-19 severity ranges from asymptomatic to life-threatening. Chronic kidney disease (CKD) associated immunosuppression and comorbidities raises concerns of potentially more severe COVID- 19, especially for end-stage kidney disease patients1. Early during the course of the pandemic, a meta-analysis established CKD as a risk factor for severe COVID-192. Observational studies, comprised mainly of hemodialysis patients, further add to the idea of increased severity and death risk3-7. However, published data on CKD patients under peritoneal dialysis (PD) with COVID-19 and associated outcomes is scarce8,9.
SUBJECTS AND METHODS
We analyzed our PD center COVID-19 cases for incidence, severity, hospitalization and death rates, comorbidities, presenting signs and symptoms, as well as persisting symptoms, from March 2020 to January 2021, before full vaccination with the Pfizer BNT 162b2 mRNA vaccine in February 2021. During this period, due to concerns of
increased morbidity, we considered the International Society for Peritoneal Dialysis recommendations10 and routine visits were substituted by regular phone calls. Access of patients with suspected exit-site infection and peritonitis, as well as those in training, remained unaltered.
Furthermore, patients were subjected to national and regional directives regarding full and partial lockdowns. Screening for COVID-19 in symptomatic and asymptomatic patients followed public health authorities’ recommendations. A descriptive statistic was carried out.
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and interquartile ranges for variables with skewed distributions.
RESULTS
There were 13 cases of COVID-19 out of 96 patients on PD (cumulative incidence 13,5 cases per 1000 patients-month), of which six were females (46,2%) with a median age of 57 years (IQR ± 23) - Table I. Autosomal dominant polycystic kidney disease was the most common etiology of CKD (30,8%) and median dialysis vintage was 42 months (IQR ± 44). Median Charlson comorbidity index (CCI) was 5, which translates to an estimated 21% 10-year survival chance.
Most frequent comorbidities were hypertension (100%) and a current or former smoker status (30,8%). There were no pharmacologically immunosuppressed patients. Nine cases were considered mild (69,2%), two moderate (15,4%) and one severe/critical (7,7%). There was one asymptomatic patient (7,7%). The most common presenting signs and symptoms were myalgia in nine patients (69,2%), cough, fever and asthenia each in eight patients (61,5%), hypotension and loss of smell and/or taste each in seven patients (53,8%). Four patients required hospitalization (30,8%) - three mild and one severe case. The main reason for hospitalization was incapacity to perform unassisted peritoneal dialysis due to hypotension and asthenia (three out of four). One 79-year-old patient, with a CCI of 10, died in the intensive care unit (ICU) with septic shock (7,7%) and 12 patients recovered (92,3%). The median time of hospitalization until discharge, or death, was 6 days. There were no episodes of thromboembolism, and we did not use any corticosteroids or experimental drugs against COVID-19. Of eleven patients presenting with COVID-19 symptoms, nine reported persisting symptoms for over one month (81,8%) - see Table I for maximum duration of each symptom. Residual renal function was unaffected by COVID-19. Of note, we have one COVID-19 death to report, four months after vaccination with the Pfizer BNT 162b2 mRNA vaccine, despite being seropositive for the spike protein and seronegative for the nucleocapsid protein ten days after the symptoms started (IgG + anti-S1 > 100 U/mL, IgG + anti-S2 > 100 U/mL, IgG + anti-RBD 43 U/mL, IgG negative anti-N < 1/mL).
Table I Descriptive statistics of COVID-19 cases in patients under peritoneal dialysis
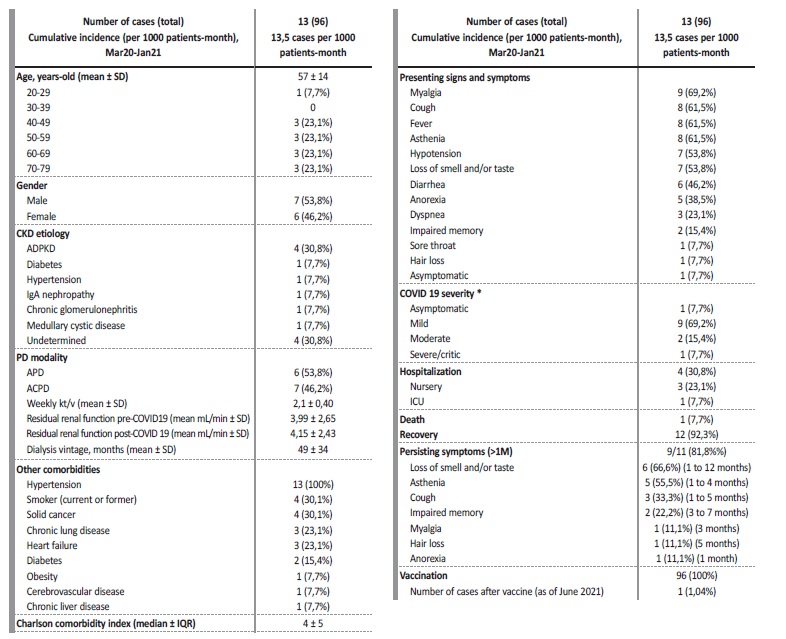
SD - standard deviation; IQR - interquartile range
* according to the Food and Drug Administration - mild (symptoms other than dyspnea), moderate (dyspnea without oxygen therapy), severe (oxygen therapy) and critical (shock or intubation or high-flow nasal cannula)14
DISCUSSION
There was a COVID-19 incidence of 13,5 cases per 1000 patientsmonth, above regional and national reported incidences for the same period (8,4 and 5,5 cases per 1000 patients-month, respectively11).
Seroprevalence studies point to an underestimation of a tenth or more of the true incidence of COVID-19, as reflected by seropositivity of previously unrecognized COVID-1912,13. Although theoretically more prone to infections due to uremia induced immunosuppression, our PD patients also have a facilitated access to healthcare facilities and testing, which could translate into a less pronounced discrepancy between diagnosed and undiagnosed cases. Since we cannot ascertain the true number of COVID-19 cases in each group, any risk extrapolation regarding COVID-19 amongst peritoneal dialysis patients would be misleading.
We considered the Food and Drug Administration criteria for COVID-19 baseline severity (Table I)14. The relative frequency of different severity disease stages was similar to the general population15, although we should note that only one patient required oxygen therapy, in the ICU. Two patients were considered moderate as they complained of dyspnea, even though without respiratory failure or need to be hospitalized. They also had chronic lung disease, were current smokers on three anti-hypertensive drugs and one suffered from heart failure. Given these comorbidities, one could question the degree to which COVID-19 contributed to dyspnea, instead of hypervolemia or structural baseline chronic lung changes.
We do not possess chest imaging of the time. As such, the majority of PD patients had a relatively benign course of COVID-19, although we should note that the median age of 57 years of our cohort was low. In fact, age is an important risk factor for severe disease, hospitalization, and death, which increases by several-fold among individuals 80 years and older compared to individuals between 50 and 59 years old16,17.
We experienced an unusual number of hypotensive patients (7/13 - 53,8%), without other organ dysfunction, requiring ultrafiltration technique and hypotensive drug adjustments. Of these, four were managed as outpatients. Hypotension is not a common presenting sign, even in critically ill Covid 19 patients (around 30%)18,19. Acute COVID-19 on chronic end-stage renal disease inflammation could account for this unusual finding, although circulatory inflammatory markers were not measured in most. We had a mortality rate of 7,7%, which is much higher than reported death rates for the general population, although the sole number of deaths in our series does not allow to draw any conclusions. This patient was the second oldest, with the worst CCI (10, corresponding to a 0% chance of 10 year-survival).
Over 80% of PD patients reported persisting symptoms for over one month, in line with other reported case series, although variable frequencies have been noted20,21. It is yet unclear if these post-COVID-19 symptoms represent a unique feature of this virus or a nonspecific response similar to other infectious diseases. Loss of smell and/or taste and asthenia were the commonest symptoms. There was no functional limitation resulting from the disease as measured by the “Post-COVID-19 Functional Status scale”22 and patients are all currently asymptomatic and without sequelae.
CONCLUSION
In conclusion, COVID-19 in patients under PD had a relatively benign course with symptoms mainly unrelated to the respiratory tract and only one case of respiratory failure and death. Unusually high rates of hypotension and hospitalization due to isolated hypotension were observed. Persisting symptoms over one month was common. In the future, it will be interesting to illustrate the degree of seroconversion and protection against COVID-19 conferred by the Pfizer BNT 162b2 mRNA vaccine in this subset of patients.















